Duloxetine: Refining its Chemical Synthesis with Biocatalysis
09.03.2014 -
Competing With Generics - With product innovation falling and drugs going off patent, the generic pharmaceutical market is becoming more predominant. It is expected that generics will be adopted in almost all major pharmaceutical markets before 2020. India and China are especially promising, because of their ability to undertake cost-effective active pharmaceutical ingredient (API) and pharmaceutical intermediate manufacturing. Manufacturers of patent-protected therapies must therefore increasingly compete with generics manufacturers.
This increased competition over products has put cost reduction at the forefront of manufacturers' minds.
A Change In Thinking
Pharmaceutical innovators can compete with generic manufacturers by using more effective manufacturing processes. Conventionally, cost reduction measures have taken place in the later stages of clinical development, but as pressure to save money has grown, innovators have increasingly employed them earlier.
Investing in new technologies can improve the productivity and efficiency of manufacturing intermediates and APIs. Biocatalysis reduces costs while still providing a sustainable process and improving API quality. By utilizing this type of method earlier, innovators can better protect themselves against future generic manufacturers and remain aligned with regulatory demands.
Employment Of Biocatalysis
Biocatalysis is the use of natural catalysts (e.g., enzymes), in place of chemical catalysts in synthetic processes. This enables new, sustainable routes of production for intermediates and APIs. It is an important tool for medicinal, process and polymer chemists, allowing the development of efficient and highly attractive organic synthetic processes on an industrial scale.
One advantage of enzymes in organic synthesis is their unique selective properties, which give a number of commercial benefits, such as better production of single stereoisomers, fewer side reactions, fewer reprocessing/purification steps and easier product separation.
Developing Duloxetine
Duloxetine is a serotonin-norepinephrine re-uptake inhibitor used to treat a wide range of conditions, including major depressive disorder and chronic musculoskeletal pain. The main brand of Duloxetine, Cymbalta, went off patent in December, making it a candidate for cost reduction.
This article will outline the specific disadvantages of chemical catalyst synthesis and the benefits of using biocatalysis enzymes during the manufacture of Duloxetine APIs and intermediates. It will demonstrate how cost savings and improved end products are achieved through improved API quality, updated synthesis routes, process sustainability and technical benefits.
Chemical Synthesis Of Duloxetine
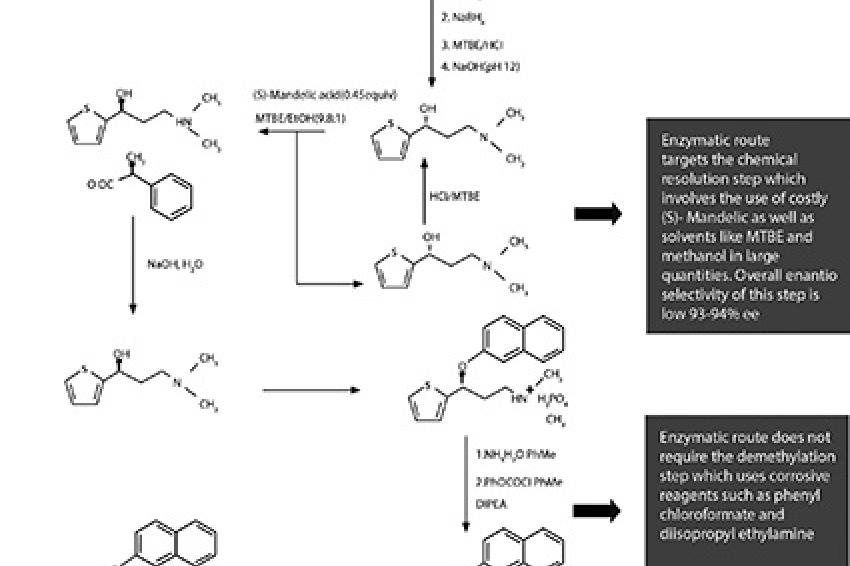
The chemical synthesis of Duloxetine is shown in Figure 1. Initially, racemic 3-(N,N-Dimethylamino)-1-(2-thienyl)propan-1-ol is formed. The mixture then undertakes resolution to form (S)-N,N-Dimethyl-N-[3-hydroxy-3-(2-thienyl)propyl]ammonium (S)-Mandelate, which is converted to (S)-Duloxetine Hydrochloride.
This method for creating Duloxetine has several disadvantages. For instance, many chemical steps are included,, increasing manufacturing cost. Extra steps are also added for the recycling of the resolving agent and isomer-recycling, which takes place by heating of the (R) isomer with hydrochloric acid. This forms impurities, which may affect the end product.
Furthermore, the final yield of this process is restricted to around 10%-12% of the starting material. A key factor in this high loss of yield is the chemical resolution of 3-(N,N-Dimethylamino)-1-(2-thienyl)propan-1-ol and the removal of one or two methyl groups of the dimethyl amino side chain. This elimination entails extra treatment of (S)-3-(N,N-Dimethylamino)-1-(2-thienyl)propan-1-ol with corrosive reagents, such as phenyl or ethyl chlorformate, and basic hydrolysis before condensation with 1-Fluoronapthalene.
Importantly, the resolution step creates a low enantiomeric excess, reducing the purity of the final product. Chemical resolution of key intermediate formation also requires the use of costly (S)-Mandelic acid in half molar quantities.
To create a more cost-efficient and sustainable manufacturing process, other methods should be considered, such as the use of biocatalysis enzymes.
Enzymatic Synthesis Of Duloxetine
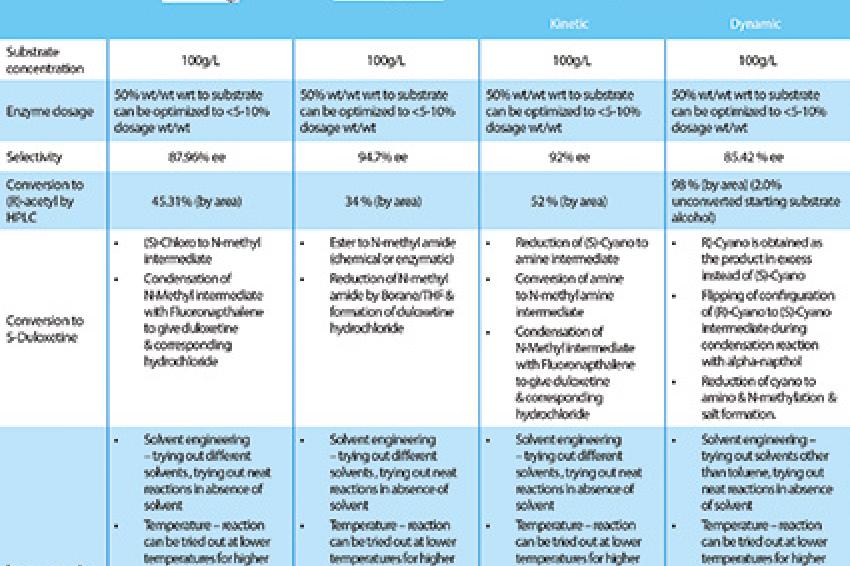
The enzymatic routes for the synthesis of Duloxetine (Table 1) show notable improvements on the chemical synthesis route.
Cost Reduction
Costs of manufacture are reduced through various methods using biocatalysis. Formulators can use enzymes to resolve an existing racemic mixture, create new chiral centers or deliver a chirally pure compound. This improves the production of single stereoisomers, creates fewer side reactions, allows easier separation of products and reduces waste. Because of the efficiency of the technique, the total cost of applying biocatalysis in drug manufacture is fairly low.
The synthesis of Duloxetine through biocatalysis is vastly cost-efficient because of the reduction in raw material input and consumption - the required components are reduced by as much as 15%. There is also a drop in overhead costs due to fewer process steps, and a reduction of up to 15% in equipment, labor and energy costs - all resulting in higher throughput and financial savings.
Sustainability
Because of the high selectivity of biocatalysis enzymes, the number of stages used during product synthesis is reduced, which in turn decreases waste production. The biocatalysis enzymes themselves can be created from renewable sources and mostly operate in water, eliminating the need for organic solvents and hazardous chemicals. Overall, the entire manufacturing process becomes more sustainable and environmentally friendly.
A Purer Product
The enzymatic process for Duloxetine synthesis uses an immobilized enzyme, providing enhanced stability and allowing convenient handling. Importantly, these can also be recycled and reused, decreasing the overall cost and improving efficiency. If required, the enzymatic synthesis route can be converted into a continuous packed column/membrane-based process.
These advances have an inevitable influence on the final product. It has been noted that biocatalysis creates more linear synthesis routes with fewer intermediates. In addition to improving productivity, this creates a better quality of API or intermediate.
The biocatalysis method creates fewer opportunities for intermediates to be passed on to the final API, making a purer product. In addition, the chiral resolution itself is very clean, so the risk of carryover of chiral impurities is small. Finally, reducing the use of solvents in the process reduces the chance of contamination.
Conclusion
Within API and intermediates manufacture, demand is rising to improve operating margins while still generating a pure final product. Manufacturers are keen to include these measures earlier in the product life cycle. As shown by the effective execution of biocatalysis in the synthesis of Duloxetine, organic enzymes can greatly benefit both final drug products and manufacturing processes.
An alternative method enables manufacturing procedures to become more streamlined and cost-efficient. Biocatalysis can create a route for companies seeking to boost sustainability, improve performance and cut costs. In this way, manufacturers are better placed to contend with generic products in this increasingly competitive marketplace.
Contact
The Scott Partnership