7 Steps to GMP Compliance
Updating Chinese GMP Guidelines - The Chinese know that implementing good manufacturing practice (GMP) is a prerequisite for a certificate but in the past, the Chinese GMP system could not be compared to Western GMP standards. The first GMP manual in 1988 focused on cleanrooms and cleanroom technology.
It appears as if the installation of such a cleanroom would constitute the whole GMP status.
Chinese authorities have emphasized the meaning of this technology by issuing special booklets detailing the technology, the qualification and monitoring, including ready-to-use forms and checklists.
Third-party audits of Chinese API manufacturer sites confirm this focus on cleanrooms. While most of the process steps were executed under unacceptable environmental conditions, the last step always was handled in the cleanroom area, which sometimes provided better conditions, as with Western API manufacturers. Only the definition of a typical Chinese 300000 cleanroom class seemed to be less restrictive compared with a Western 100000 cleanroom class - even the air quality was comparable. And since all of the production plants in China are designed by official local design institutes, this situation could be found all over the country.
But the Chinese learned quickly, and the government noticed the failure to comply with worldwide pharmaceutical quality standards. Task teams have been set up to adapt the Chinese GMP guidelines, and groups of SFDA (Chinese State Food and Drug Administration) inspectors have been trained on the Western GMP requirements including but not limited to the escorting of Western official inspections.
These activities finally resulted in the Chinese GMP guidelines valid as of March 1, 2011. A booklet comprising 14 chapters, 313 articles and five annexes describes the Chinese GMP system. It's very similar to the Western GMP standard but not exactly the same. As one example, the starting point of GMP in an API production process is addressed but still not finally defined as this is done in the ICH/Q7 GMPs.
Other more modern topics like quality management and risk management are integrated and emphasized and even the 300000 cleanroom class is adapted to the 100000 one. The main text as well as the five annexes is available in English but not yet as an official translation. The timeline for adapting to the new regulations is December 2013 for special (e.g., sterile) products and December 2015 for all others.
Auditing Quality into the Product
A basic and substantial philosophy of GMP is "not to test quality into the product but to build it into the product." That is why a pharmaceutical manufacturer has to focus on all items potentially influencing the quality of the final drug products, for example, personnel, buildings and equipment, starting and raw materials, processes, and in-process controls.
In addition, when it became obvious that the quality of the related API is essential, the authority started to force the final drug product manufacturers to buy APIs only from GMP-compliant manufacturers. The well-known and related EC directive 2004/27 caused a rush of audits by the final drug product manufacturers at different API sites. As far as those sites have already implemented GMP and as long as the audit has the intention only to verify the functionality of the system, this procedure may be accepted. If the auditee has not yet implemented GMP or the GMP system is very poor, an audit only can identify this fact and stop the business as long as GMP is not correctly set up.
If audits are executed only to salve one's conscience, this is not acceptable and even repeated audits are to be interpreted only as "auditing quality into the product."
China, which is the No.1 source for APIs, still suffers from many API manufacturers not complying with GMP - especially Western GMP. Many audits in China in the past years have had more or less destructive results. And many manufacturers don't know how to interpret the western GMP requirements and how to fill the gaps in compliance. They just wait for the next audit and hope to survive.
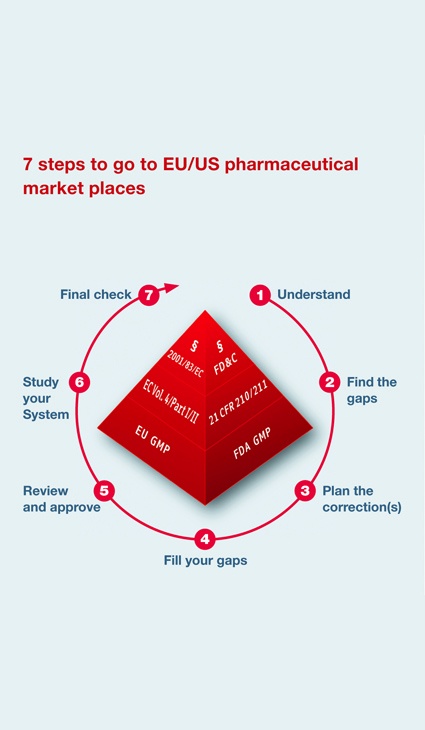
Seven Steps to GMP Compliance
So far, audits alone in China will not be sufficient to assure compliance with Western GMP standards. More activities are required for a long-term reliable and good quality API source or even for a location for final drug product manufacturing.
- Step 1: Provide the necessary transparency means to make clear to the manufacturer the differences between Chinese and Western GMP standards. As long as they don't know this in detail, there is no chance to improve the system. Training or even some simple consultancy could be the method of choice. It is the basis for all further activities.
- Step 2: Analyze the gaps of the existing system. Experts or trained auditors are necessary to do this successfully. Also time is needed, as it is not to compare with an audit. Systematic reviews of the different GMP-related systems are required. This includes onsite reviews as well as intensive document reviews.
- Step 3: Install a project management plan to focus on all findings and gaps revealed in step 2. Setting priorities and timelines is essential and should be agreed upon by the related manufacturer. Without such a project management plan, the chance to reach the said goal in China is very low.
- Step 4: Improvement and CAPA (Corrective and Preventive Action)activities.
- Step 5: Adapt most important systems, such as validation or risk management, to Western standards. SOPs must be processed or adapted to the individual needs and structure of the manufacturer's site. Steps 4 and 5 are the most time-consuming and need the most supervision and follow-up.
- Step 6: Train all employees, from basic staff up to high-level management. This will help assure transparency with the new system.
- Step 7: Execute a mock inspection by a Western expert or educated auditor to determine whether the "GMP upgrade" was successful.
Key factors for success are the involvement of experienced Western experts as well as local Chinese experts who bring together Western GMP experience with deep knowledge of cultural and language-related aspects. Neither Western nor Chinese experts can do such a challenging job alone.
Moving Forward
The Chinese pharmaceutical industry is rapidly growing and is on the way to overcoming its problems with outdated GMP standards. The national authority - the SFDA - took the first step in this direction when it issued the 2011 GMP guidelines, which are close to EU standards. Manufacturers still need time to catch up with the new requirements, and simple GMP audits are not the best way to support this. A more detailed and target-oriented program, such as the seven-step procedure outlined here, is necessary. And Western companies cannot successfully execute these as remote programs but need the help of Western and Chinese experts in combination.
The Old Philosophy
Gempex joined the Chinese CPhI exhibition in Shanghai for the first time in 2005. Our booth was small because the company had just been founded and the budget was small.
Our simple banner listed keywords dealing with good manufacturing practice (GMP) and drug master files. Even if it was not the most attractive booth, the result was unbelievable. Hundreds of visitors pressed around the banner, studying those words like a bible. My partner later called it the "$10,000 banner."
But the final result after this impressive response was disillusioning. No real job came from the three days in Shanghai because for all the visitors there was one important question: "How can I get a Western GMP certificate?"
At that time, there was no real understanding of the Western GMP system and the activities behind it. Chinese pharmaceutical and active pharmaceutical ingredients (API) manufacturers thought it should be easy - as it was in the Chinese GMP system - to get a GMP certificate to open the Western pharmaceutical market for them.
We joined the CPhI year after year, and it was interesting to keep track of how the situation changed. One year later it was not only the question of the Western GMP certificate but also of how to fulfill the related GMP requirements. How to fulfill the "real" GMP was the question of the following year. Some years later more and more visitors asked sensitive questions about the differences between Chinese and Western GMPs and what to do to overcome the gaps. And we end up with the question: What has to be done in order to receive an official GMP certificate?