Disinfectant Residues
Mitigation and Management
Many common and well used disinfectants, leave significant residues on a surface, which can subsequently have a detrimental effect on the effectiveness of the disinfectant used. This is acknowledged in the new GMP Annex 1, “For disinfection to be effective, prior cleaning to remove surface contamination should be performed. Cleaning programmes should effectively remove disinfectant residues.”
Cleaning and disinfection are two words that have often been used interchangeably, when in fact, they have two different meanings. Annex 1 now clearly states the difference: A process for removing contamination e.g. product residues or disinfectant residues. Cleaning is physically removing nonviable matter from a surface. Disinfection: The process by which the reduction of the number of microorganisms is achieved by the irreversible action of a product on their structure or metabolism, to a level deemed to be appropriate for a defined purpose. The reduction in the amount of viable contamination on a surface. Residue removal is specific to cleaning.
There are currently no approved or validated methods for assessing the amount of residue on a non-product contact surfaces. Many or even most facilities will conduct a visual test for residues on non-product contact surfaces only. As previously mentioned many of the commonly used disinfectants in cleanrooms, can themselves leave significant residues on a surface. Disinfectant residues can cause visual, safety, and product integrity threats, including sticky or slippery floors and doors, streaks and discoloration, and contamination. If residues are not managed correctly, they can also cause degradation to the facility over time, which can lead to costly reconstruction or require deep cleaning measures.
Application of disinfectants and proper use of tools such as wipes and mops play a critical role in residue management and there are many variables that can pose issues when cleaning and disinfecting including over-application of disinfectants, frequency of residue removal, reapplication of disinfectant (if longer contact times are required), and the nature of the disinfectant. There is no one-size-fits-all approach for mitigation of residues as part of an overall Contamination Control Strategy (CCS).
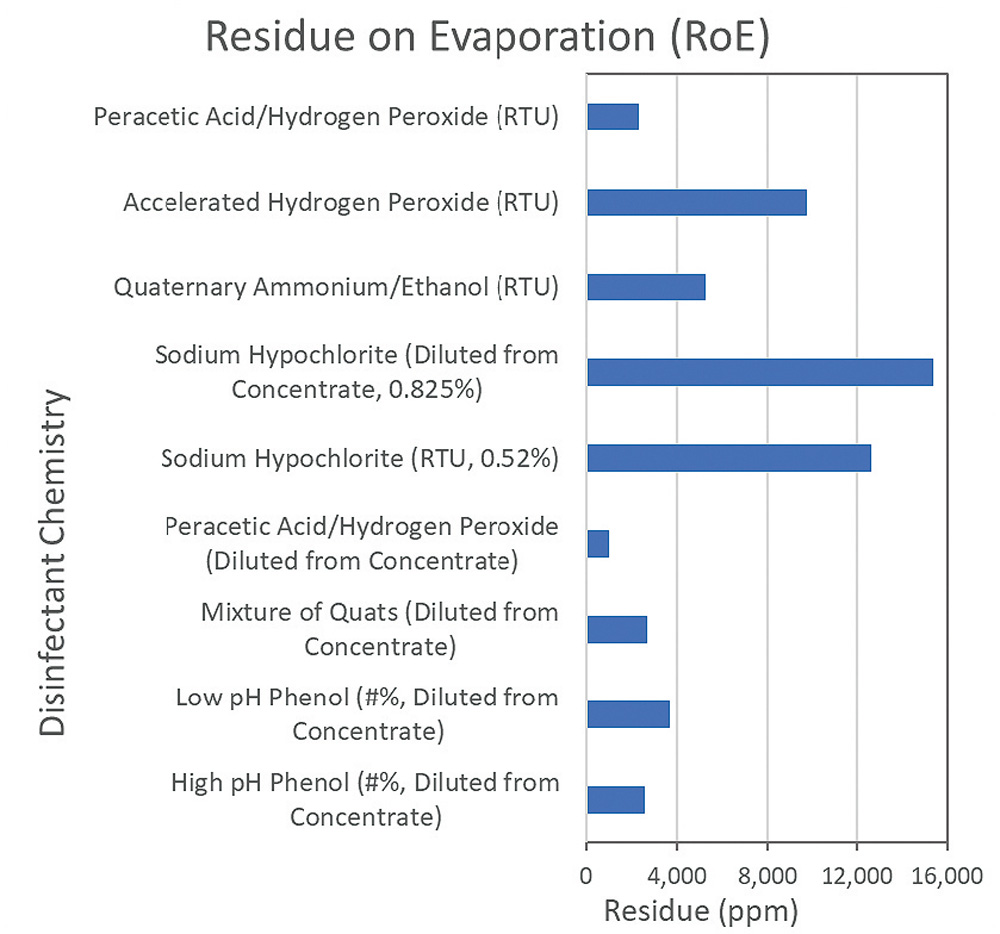
An initial thought maybe to use a low or no residue disinfectant to solve this particular issue. There is currently no definition or standard of what constitutes a “no” or “low residue” disinfectant. However, there are claims made on disinfectant advertising that a disinfectant is either “no” residue or “low” residue. The data to support this is usually a simple residue on evaporation test.
The European Pharmacopoeia has a residue-on-evaporation test (RoE) which can easily and cheaply be used to quantify the amount of non-volatile residue left by a solution. The test method simply requires 100 ml of the solution to be boiled to dryness in an evaporating basin of known weight.
EP Residue on Evaporation Method
- Evaporate 100 ml of test substance to dryness in a water bath and dry at 100–105 °C for 1 hour
- Weigh container after drying and subtract weight of the original container
Two chemicals used as disinfectants which do have an EP monograph are Isopropyl Alcohol and Ethanol, these have limits for residue on evaporation. The limit for 99% Isopropyl Alcohol is 20 ppm and 25 ppm for 96% ethanol. Both of these products would be universally accepted as leaving no residue on a cleanroom surface. So, a product which leaves a non-volatile residue of less than 25ppm could be classed as no residue ?
The only other commonly used cleanroom disinfectant which leaves a residue as low as alcohol is hydrogen peroxide. Hydrogen Peroxide (H2O2) breaks down to water and oxygen on a surface, the reaction taking place is 2H2O2 → 2H2O+O2. Test worked carried out on Contec’s Hydrogen Peroxide confirms this with ROE results between 4 ppm and 7 ppm. However, this cannot be assumed for all hydrogen peroxide solutions. It is a solution in equilibrium so different grades of hydrogen peroxide contain different amounts of stabilisers which can contribute to the amount of residue left behind.
Blended disinfectants which contain alcohol or hydrogen peroxide with other chemicals can also leave considerable residues. Table 1 shows the residue on evaporation levels for a range of cleanroom disinfectants manufactured in both the USA and Europe. As can be seen from the results, the level of residue for the different active ingredients can vary significantly from product to product. The table also shows there can be a difference for disinfectants containing the same active ingredient, which would be due to the concentration of the active ingredient in the product, the amount of stabilizers required, and potentially the addition of preservatives, pH adjusters or odour mitigators.
There are drawbacks to both of these disinfectants which means other disinfectants may have to be considered. 70% alcohol is highly flammable so cannot be used over large surface areas, and also is not sporicidal. Hydrogen Peroxide is an aqueous based solution which is non-flammable but at a safe to use concentration of 6% only has a low level of sporicidal activity in 60 mins.
Cleanroom disinfectant residue on a surface
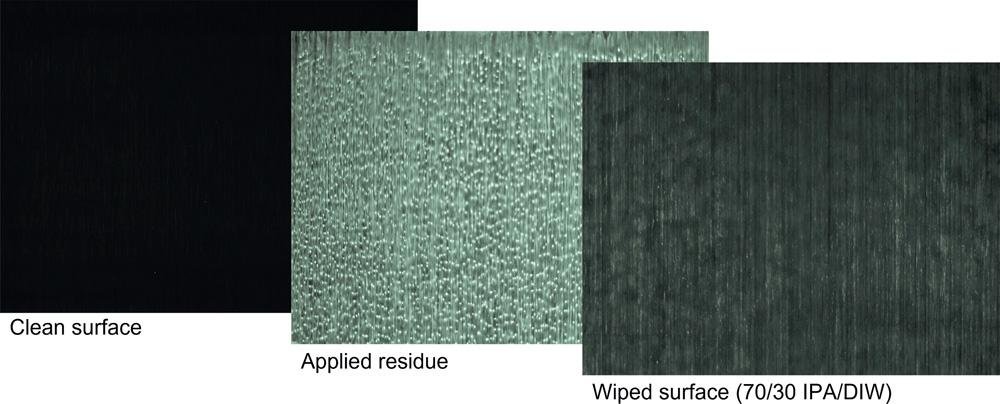
Disinfectant residues can take many forms, clear, white, yellow, pink, solid, gelatinous, crystalline, powdery, or sticky. Work carried out by Contec (ReinRaumTechnik Mar 2021) showed that the visual appearance of the disinfectant on a surface may not always match the amount of residue shown on a residue on evaporation test. The method of application of the disinfectant will also affect the residue.
The appearance of disinfectant residue on different surfaces within the cleanroom can look lesser or greater for the same amount of residue dependent on the characteristics of the surface itself. Highly polished or reflective surfaces will show residues more easily. It is common to see disinfectant residues on windows, which can make a facility look dirty and unkept. Highly reflective surfaces, such as glass and polished metals, will show the residue more significantly, although the amount of residue will be the same.
Is there a better way than visually clean?
The current accepted validation for a non-product contact surface being clean, is a visual inspection. Within ASTM-E3263 “Standard Practice for Qualification of Visual Inspection of Pharmaceutical Manufacturing Equipment and Medical Devices for Residues” there are suggestions to make the comparison between operators of “visually clean” as repeatable and robust as possible. We wanted to find out if we could find a correlation between visibly clean and a quantifiable metric, and if we could make that quantitative method of assessing residues, easy to use and robust for normal use. We carried out work using various methods to quantify residues and residue removal, a gradation scale for visual assessment, using a haze meter on mirrors — a sort of Finite Element Analysis and finally a fluorescent tracer and image pixel analysis.
Using a visual analysis and a graduation scale had some benefits as even slight residues were easily discernible. We were still left with the residue being difficult to quantify as it was very subjective and operator dependant. An attempt to measure surface cleanliness using a Haze meter on residues on mirrors was also very objective and time consuming. The residue was easy to quantify but it was objective. Though overall results could be useful, random and trace residues that were easily visible were not captured and reflected in data. It highlighted the sensitivity of the naked eye to even minute levels of residue.
The residue field was variable and the results inconsistent with visible trace residues. The best results were obtained with the fluorescent tracer and image pixel analysis.
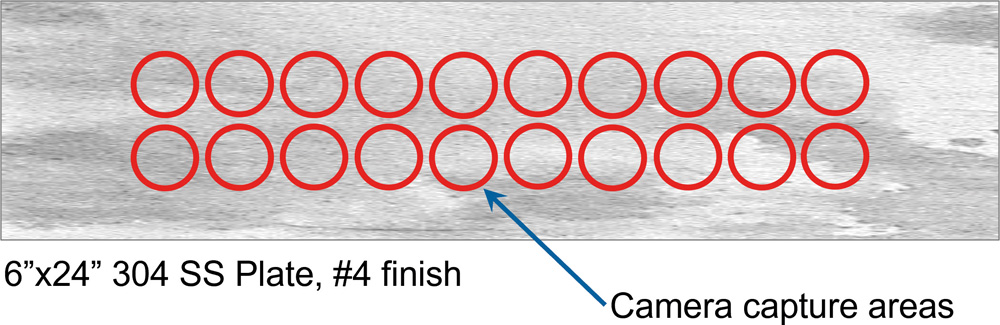
A fluorescent tracer dye was added to average-residue disinfectant and applied by wiping. Each application was allowed to dry before an additional layer of disinfectant, and therefore residue was applied. A camera pixel analysis was used to measure the surface residue. This was easy to quantify and the full residue field could be analysed with the image pixel analysis. The results obtained were consistent with the visible trace residues. It could be easily conducted on a wide variety of surfaces. The initial test work was carried out on 150 x 150 mm stainless steel plates with 3 x 50 mm diameter image analysis areas. We found that the disinfectant residues applied are very irregular and non-uniform (splotchy!) probably due to the surface tension interaction with the coupon surface. Because of this, the image analysis area was not always sufficient to give results with accurately corrolated with what was visually observed on the coupons.
Additional work was carried out on coupons which were 150 mm x 610 mm with 18 x 50 mm diameter image analysis areas. (Fig. 2) The much larger image analysis area gave results that more accurately correlated with what was visually observed on the coupons. Understanding that disinfectant residues are very irregular and non-uniform due to surface tension interaction with the surfaces explains why the quantification of the distribution and the total disinfectant residue can be very difficult.
Not all residues behave the same
As table 1 showed many common disinfectants leave a residue to greater or lesser degrees. Annex 1 states that the cleaning process should be validated so that it can be demonstrated that it can remove any residue that could create a barrier between the decontamination agent and the equipment surface. But not all residues behave in the same way so consideration should be given not just to the amount of residue left but how easily the disinfectant residue is to remove from the cleanroom surfaces. Old and scratched surfaces may also influence residue removal. Some residues are free-rinsing and easy to remove, others are “sticky” and waxy and can be difficult to remove from a surface. This can also change over time, a residue easily removed immediately after the contact time might not be so easily removed weeks later. If an immediate rinse stage is not to be used, work would need to be carried out to show how long a residue can be left before removal becomes more difficult.
Validation factors
This starts to give an insight into how much validation work may be required to meet the requirements of the new Annex 1 if a “no-residue” disinfectant is not being used. A lab based, residue-on-evaporation test, will give a quantitative result of how much residue a disinfectant will leave and if it is above 25 ppm per 100 ml further validation work would be required.
The method of application of the disinfectant will need to be taken into account, as this affects the amount of residue left on a surface in use. The work needs to be carried out on the different surfaces in the cleanroom as the appearance of the residue on the different surfaces varies dependant on the smoothness and reflectiveness of the surface.
If an immediate wipe to dry or rinse stage is not used at the end of contact time, the amount of time the residue can be left before removal needs validating as a residue that can be removed after a week may not be able to be removed after a month. The roughness of a surface will also influence how easily a residue can be removed. The choice of cleaning solution will need to be validated, whether WFI, alcohol or a detergent (surfactant) solution works best. This might not be the same for all disinfectants that you are using.
Controlling disinfectant residues
One of the simplest ways to control the disinfectant residue is to not let it build up in the first place. This could be achieved by changing the cleaning and disinfection SOPs so that a wipe-to-dry phase is included. Immediately after the validated contact time for the disinfectant wipe the surfaces to dry with either a dry wipe or mop head.
Alternatively, a disinfectant residue removal step is necessary to mitigate the residue and maintain cleanliness of the surfaces within the cleanroom. It could include the use of 70% alcohol solution or presaturated wipes, Water for Injection or Purified Water, or 6% Hydrogen Peroxide. This could be a weekly or bi-weekly removal of disinfectant residues either immediately after the validated contact time or prior to the next disinfection application. If using alcohol for residue removal, the Health and Safety issue of alcohol use over large areas would need to be considered.
If residues are allowed to build up, a regime for a detergent / solvent clean on a monthly or quarterly basis could be considered. If the point at the which a residue ceases to become free-rinsing is known then this would govern the point at which the detergent clean became necessary. As all detergents leave residues this would be a 3-stage process of clean, remove detergent residue and wipe to dry. So, this may not be the least time-consuming option it appears to be. The cleaning agent might need to be more aggressive or corrosive than if the residues are removed more frequently. Whichever decision is taken it will need to be captured in the Contamination Control Strategy, based on risk management and monitored through environmental monitoring.
Evaluation of residue removal protocols
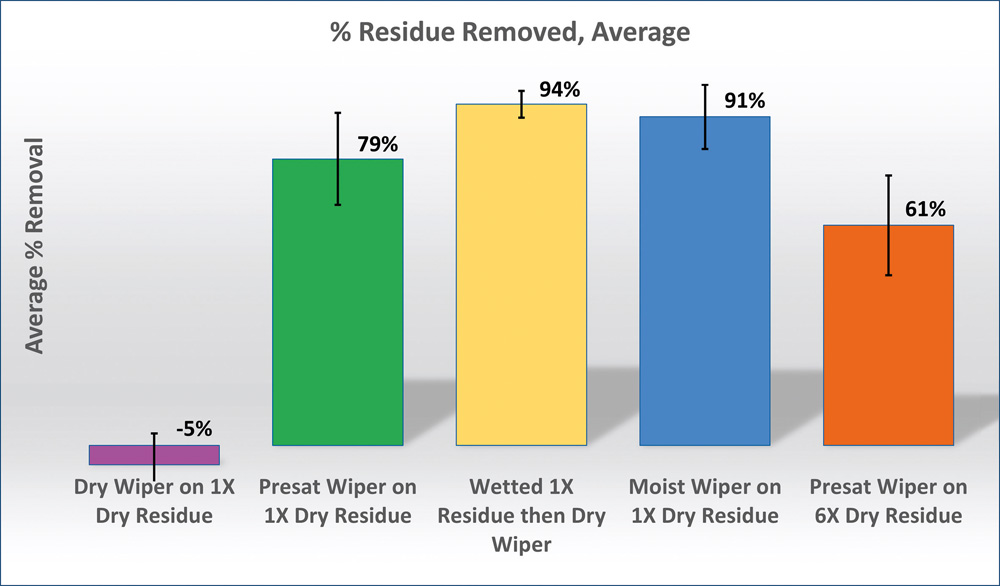
We used the fluorescent tracer and image pixel analysis to see if we could see a difference in the residue removal protocols. Eighteen sampling locations were used per replicate and 5 replicates per trial. A fluorescent tracer dye was added to average-residue disinfectant and applied by wiping. Each application was allowed to dry before an additional layer of disinfectant, and therefore residue was applied. The following procedures were compared.
- Dry residue, wiped with a dry wipe
- Dry residue, wiped with a 70 % IPA
- presaturated wipe
- Dry residue, sprayed with 70 % IPA solution,
- then wiped with a dry wipe
- Dry residue, wiped with a moist wipe
- Six layers of dry residue, wiped with a 70 % IPA presaturated wipe
Pixel analysis was carried out on the clean coupon, after the residue was applied to the coupon and after the coupon had been wiped/sprayed etc. The final calculation was a comparison of average % residue removed.
It will not necessarily come as any surprise but it can be seen in Table there was a significant difference in the ability to remove a dry residue with a dry wipe versus a presaturated wipe. Solubilising the residue also allowed more of the residue to be removed with a presaturated wipe than a presaturated wipe used on the dry residue. The most effective result was gained by solubilising the residue and using a dry wipe, a similar result could probably be obtained but removing a still wet disinfectant solution with a dry wipe after the contact time. Again, confirmation of what we expected to see was that residue accumulation greatly increases the difficulty in removing the residue from a surface. Further work could be carried out to see if these results are consistent for all disinfectant residues.
Conclusion
Disinfectant residues can pose significant cleanliness and operational risks in cleanrooms and other controlled environments, in additional to degrading the appearance of the cleanroom and suggesting a lack of control. Regulatory organizations worldwide recognize the need to remove or otherwise mitigate disinfectant residues as part of an effective overall Contamination Control Strategy. The recently published Annex 1 specifically states that “Cleaning programmes should effectively remove disinfectant residues.”
Acknowledgements
Thanks to NA and EMEA Technical Services Group for all their hard work on residue analysis. Dave Nobile, Neil Simpson, Dr Mark Wiencek, Lauren Pernot and Chip Burnett.
Author: Karen Rossington, Contec






