A Shaky Platform?
Sourcing of Chemical Raw Materials for Pharmaceutical Industry
Monitoring The Supply Chain - The globalization of chemical raw material procurement is presenting significant challenges to the pharmaceutical industry. Adopting basic good manufacturing practice (GMP) and environmental, health and safety (EHS) standards constitutes an important tool for reducing the inherent risks. Observation of the latter, in particular, is getting more and more important. A dilemma between financial objectives and ethical behavior is particularly obvious in China and India.
A more rigorous monitoring of EHS both at manufacturing sites and during transportation of goods is essential for securing a reliable supply chain.
Introduction
In the early beginning of manufacturing active pharmaceutical ingredients (APIs), the situation was different from today. Take acetylsalicylic acid (aspirin) as an example. Aspirin was manufactured from simple and readily available chemical raw materials within two synthetic steps.
Chemical raw materials were defined as organic chemicals that could be used in organic synthesis to manufacture APIs or fine chemicals.
Chemical Supply Chain in Pharmaceutical Industry
At the beginning of the last century, the main sourcing aspects for an API manufacturer with regard to its chemical raw materials were:
- Only a few chemical steps were necessary in order to manufacture the final drug.
- Organic chemistry was booming.
- Companies such as BASF, Bayer, Dow, ICI and Hoechst were expanding their technical capabilities and their business.
- Transportation of organic chemicals took place over short distances and was well-organized.
- Regulations for current good manufacturing practice (cGMP) were not established and were virtually unknown.
- Organic chemicals were commercially available and could be considered partially as commodities.
As a result of these six major developments, the term "supply chain" has become an integral part of the sourcing process in the pharmaceutical industry, too.
As the active ingredient of new drugs became sophisticated (e.g., aspirin, launched in 1899, two steps; Pradaxa, launched in 2012, 15 steps), the pharmaceutical industry quit total backward integration and started sourcing intermediates of higher complexity.
As a consequence, a new product category, namely fine chemicals, emerged, and thus a whole new industry developed.
During the past 20 years, a very competitive fine chemicals industry developed in Asia, particularly in China and India. An important aspect of this globalization is the "Total Cost of Ownership," which has been discussed in "API Manufacturing Facts and Fiction." In connection with this shift to Asia, other serious problems in the sourcing process have arisen.
Having this new challenge in mind, the International Pharmaceutical Supply Chain Consortium Rx-360 was founded in 2009. Its mission is to enhance the security of the pharmaceutical supply chain, e.g., by both standardizing and rationalizing audits of suppliers.
Global Aspects for API Manufacturers
The situation for API manufacturers has changed dramatically within the last 20-30 years:
- Many Western organic chemical companies closed their plants and companies in China and India entered this business.
- Transportation from Asia to Europe needs more time than transportation within Europe (e.g., sea transportation from Asia takes five to six weeks). A good supply chain has to be in place in order to avoid "out of stock" risks.
- Some other risks can be added: workers going on strike; natural disasters, such as earthquakes; political riots; arbitrariness of local authorities; insufficient protection of intellectual property rights.
- Clear communication is a must to avoid misunderstandings caused by culture differences.
As mentioned above, these shortcomings may drive the "total cost of ownership" into unfavorable dimensions. Management has to take into consideration that some disadvantages of globalization cannot be well-controlled without a new sourcing strategy.
Audits And Inspections
In the course of the sourcing exercise for API or advanced intermediates, quality audits are generally conducted according to ICH Q7A.
EHS (Environmental, Health and Safety) audit has been added lately to comply with the responsible-care principle. However, these EHS audits raise fundamental questions, namely checking for compliance with local regulations only or with Western standards? Should the audits be carried out by EHS specialists or purchasing generalists? Confronting with the deficiencies of the underlying philosophy raises a "conflict between (their) company´s ethic and its financial objectives," said Tony Scott of the European Fine Chemicals Group.
Examples of fundamental parameters - though not always mentioned in reports - are difficult to assess, for example:
- Qualification systems including design, installation and operation qualification (DQ, IQ and OQ).
- Process safety management: e.g., process design, process safety studies, determination of critical parameters, risk management studies.
- Qualification and training of workers.
Supply Chain from Clinical Phase I to Market Launch
As soon as a new drug enters phase I, the sourcing aspects for the chemical raw materials have to be reviewed.
Pricing/quotations within phase I don't play a dominant role, but it is getting more important later on in case the API is on track and is becoming a bulky product.
The best option would be a "one-stop shop," where the supplier provides multiple services starting with the lab trials, scale-up to market launch with all "requirements/services" behind.
If the estimated demand for the API is less than 100 kg per year, the situation will be different. The following discussion is focusing on high-volume API requirements.
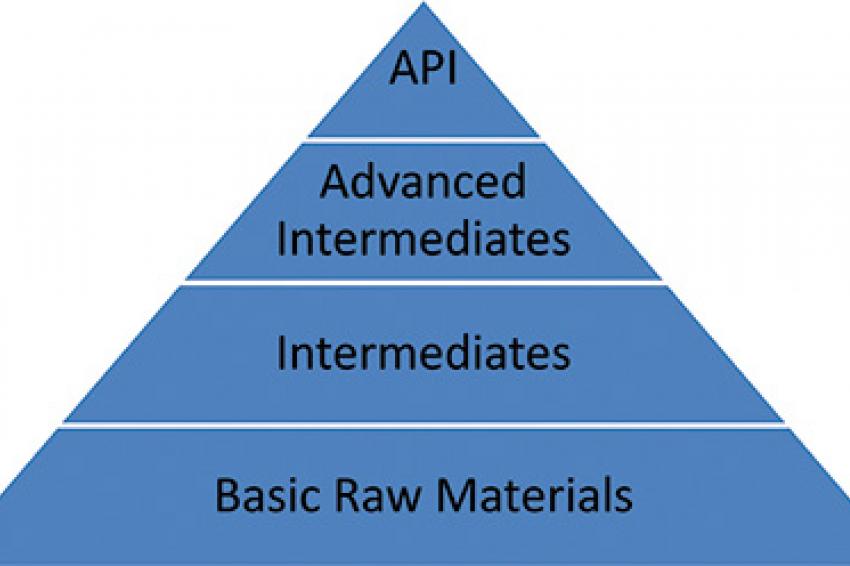
Regarding the supply chain of, for example, a 10- to 15-step synthesis, a rough classification for the supply chain for the chemical raw materials can be made, as shown in figure 1: namely basic raw materials, intermediates (IM), advanced intermediates and the final API. Note: If the wording "chemical raw materials" or only "raw materials" is used, a clear definition is needed.
The following sections are based on the definition of figure 1, which can help to get a clear understanding for chemical raw materials in general.
Advanced Intermediate And API
The regulated part of the synthesis begins normally from advanced IM. Advanced IM and the API have to be manufactured according to cGMP rules. These steps often will be manufactured by the pharmaceuticals company itself (in-house manufacturing) or by custom manufacturing (outsourcing). The custom manufacturer for advanced IM and API should be strictly controlled by the pharmaceutical company. Reliable standards for EHS and cGMP should be the minimum requirement.
Intermediate
If an IM is commercially available from the market it will be the best case from an economic point of view. It is acceptable that intermediates are produced under non-GMP conditions. The term non-GMP is misleading for several reasons. It should be replaced by basic GMP. Basic GMP is not officially defined, but it's a much better wording than non-GMP.
The expectation for a basic GMP is summarized in a table (see attachment 1). Basic EHS requirements are also included in the table. This table is a useful tool during chemical/technical inspections in the manufacturer's plant.
Here the first advantage can be seen: A thousands-ton-year that is used in the regulated part of the API synthesis, normally is not prepared according to cGMP, but often following basic GMP requirements.
Basic Raw Materials
Basic raw materials are normally commercially available and usually not regulated. The chemistry behind it is, e.g., nitration, chlorination and fluorination of aromatic compounds. Nobody is really interested in any inspection of those "nasty" chemistries. What is more, even any "observation" is not easy to arrange, since these suppliers, as mentioned before, are located in Asia. Meanwhile, Western companies would like to get best pricing together with Western EHS standards. These two targets don't match.
Suppliers of basic raw materials can constitute a risk. The following questions should be clarified in advance:
- Do they have a valid permit in place for running the plant?
- Do they have appropriate technical standards for their chemistry?
- Do they have reliable safety investigations for the process?
- Do they know the borderlines of the process?
- Do they have an acceptable/reliable EHS standard?
- Do they have appropriate management structure; are decisions focused on the owners of the company?
Purchasing people should bear in mind that the low price of the product often does not match with the chemistry behind it. Sometimes this is also a result of their target-setting and focusing on cost.
On the other hand, Big Pharma is facing serious cost problems once its top-selling originator drug becomes off-patent. In addition, costs for R&D and administration are still high and cannot be adapted on short notice.
Knowing the Real Supply Chain
It has to be mentioned that in the following discussion whether an organic chemical is a basic raw material, IM, or advanced IM is not the subject of this article.
A second challenge can be added: Pharmaceutical companies would like to change from a single sourcing strategy to a second supplier strategy for their intermediates in Phase II. The reason: If one supplier fails or in case of emergency, they are able to switch to the second one. Furthermore, they create competition for pricing.
If both supply chains for the IM are based on one basic raw material manufacturer, meaning a single source for the basic raw material, the risk may be high. (See figure 2.) Often IM suppliers are sourcing their basic raw materials from subcontractors, which constitutes an additional risk element because these companies depend on the IM manufacturer regarding orders and capital for their investment.
In the basic raw materials industry, authorities can withdraw permission/ license of the production itself. Particularly in China and India, it's sometimes not predictable what can happen.
This should be checked carefully by pharmaceutical companies when discussing the whole chemical supply chain.
What can be a solution for the manufacturers of these basic raw materials: Look for mergers or shut down their plant when realizing the need to make a major investment in order to provide better/safer technical standards?
Packaging And Transportation
When sourcing chemical raw materials from Asia, further topics have to be clarified before ordering:
- Is a simple polyethylene bag suitable in case the chemical material is a solid?
- Does the material need to be protected against light and moisture?
- Is a fiber suitable as a secondary packaging material, or is an iron drum necessary?
- Can the drum be filled to its maximum? Half filling is a matter of waste.
- Are coated drums (or drums with inliners) necessary if the chemical material is a liquid? Can the liquid be solidified? Is the drum or inliner suitable when the material has to be melted before further use?
- Are all the required packaging materials available in Asia or do they have to be imported at high price?
- Does the labeling meet international standards and internal requirements of the customer's company?
It also should be checked carefully to make sure all "transportation papers" are available and match exactly with the material itself. Pest control of the container, for example, sometimes is done before charging the material, but that is difficult to check. Storage of chemical materials at harbor for a long time can cause degradation. Thus analytical results from China and India may be different from those the customer gets after several weeks of transportation. In a worst case scenario the material is out of spec and causes the delay of the chemical production.
Conclusion And Outlook
Today's sourcing of chemical raw materials from phase I onward should be part of well-organized supply chain management in the pharmaceutical industry.
Fine chemicals are the most important category in the pharmaceutical industry, covering the line from basic raw materials to intermediates and advanced intermediates up to API.
Inspection of the manufacturer of non-cGMP products should be integrated in the supply chain. Instead of non-GMP, basic GMP (and basic EHS) requirements are defined and proposed and may become the basis in an inspection. By applying those rules, the whole supply chain is covered by basic GMP and cGMP, which may increase the security in the production process of an API.
Further risks are discussed if the start of the supply chain is unknown, and a single source is established. Inspection should start from basic raw materials because they sometimes bear high risks regarding their availability.
Price cutting by Western companies will create further risks, because basic raw materials manufacturers in Asia should get the chance to invest in better EHS standards. It would be much better to skip all extended EHS audits by Western companies and transfer this task to local authorities. Local authorities should have the biggest interest in establishing appropriate EHS standards. Corruption could be a hurdle, but it will be a better approach than a faked EHS inspection based on Western standards.
Having these facts in mind, the pharmaceutical industry should avoid listing all its outsourcing information, especially their suppliers, in a new drug application.
"Total cost of ownership" will also be a never-ending topic of a good and realistic supply chain for today.
__________________________________________________
Attachment 1: Basic GMP
Expectations on the manufacture of the
nonregulated part of the API production
(Basic GMP, Basic EHS)
General Aspects
- Permission from authorities to run the plant should be in place.
- A basic (simple) SOP system should be in place.
- Who is responsible, in general, for the housekeeping etc.
- Appropriate qualification and training program of the staff should be available.
- Documentation of master batch records and analytical methods should be in place.
- Process changes should be documented and communicated.
Cleaning Procedure: Limits can be calculated via reactor volume if compounds are toxic. For example, the limit should be 1mg/l reactor volume. If the compound is less toxic, a limit of 10mg/l reactor volume also can be accepted.
Recommendations:
- A rinse with solvents used in the process before starting production also can be helpful.
- A visual check should also be performed and noted in the documents.
Raw Materials, Intermediates, Final Products, Warehouse
- Analytical methods should be documented (also changes).
- Documents should be stored at minimum for three years.
- Clear labeling should be done for substances, also for piping, etc.
- Released and not released material should be differentiated on the packing (drums, etc.) and in the warehouse. The warehouse should be suitable to protect the material from dust, heat, etc.
Cross-Contamination
- A good, described workflow (organization plan) can avoid cross-contamination.
- Technical equipment should be suitable to perform this. Cleaning records should be kept for reference purposes.
Maintenance/Calibration
A maintenance program for the production unit and analytic department is helpful. This has to include a calibration program for production balances, thermometer, etc., and should be documented.
General Guidelines for EHS (Environmental, Health and Safety) Should be in Place
- Workers in the plant should have the skills for their work. They should wear suitable clothes and protective goggles, suitable shoes, gloves when working with toxic materials, etc.
- No problems with labor unions.
- Document where all the wastes goes. Especially water: an own wastewater treatment in place or a central wastewater station? Limits should be known.
- The fate of solid residues should be known, for example, disposal or incineration.