Hot Melt Extrusion Technology (HME)
Growing importance in Pharmaceutical processing
Modern Manufacturing - HME has been on a successful path in the pharmaceutical industry for solubilization and bioavailability enhancement, particularly regarding poorly soluble drugs.
HME Evolution
In other industries, such as plastic, rubber and food products, this technology has extensively been used since the1930s. About 20 years ago, the pharmaceutical industry began to employ melt extrusion as a promising solution for its solubilization of almost insoluble drugs. More than 100 articles have been published in scientific literature and the number of HME patents has increased tremendously- mainly in the U.S., Germany and Japan. It is easy to understand why the pharmaceutical industry invests in this process, since it has many advantages; no organic solvents or water are needed, shorter and more efficient processing times can be achieved, as a result of the process solid solutions can be very stable, and solubility and bioavailability of poorly soluble drugs are improved. The only relevant disadvantages are that HME may not be applicable for heat-sensitive active ingredients, they must be almost moisture free, and it requires investment in equipment and training (although the company can decide on third-party production with a firm that already possesses the necessary equipment and expertise).
Solid Solutions
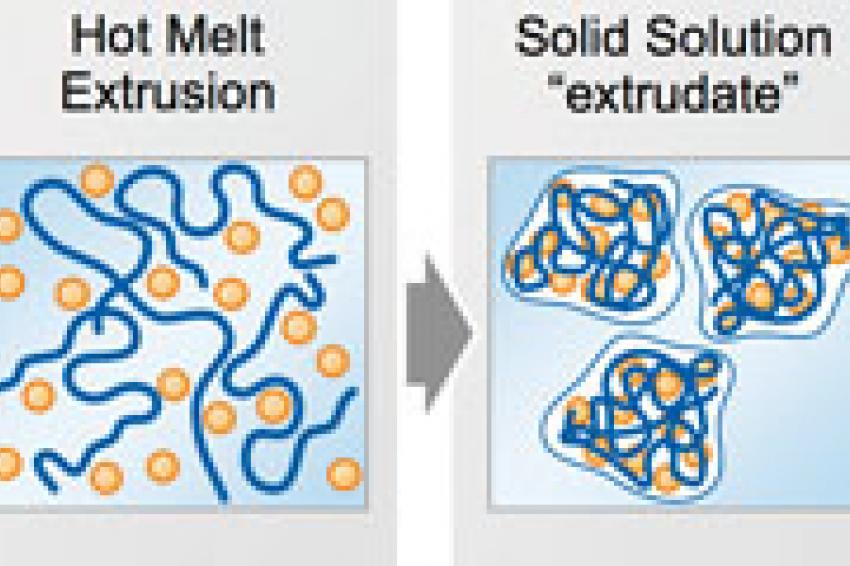
Solid solutions are similar to liquid solutions, whereas the matrix is not a liquid, but a solid. The most reliable and stable system is achieved when the drug is molecularly dissolved below the saturation point.
The practical benefit of having stable solid solutions with the drug being molecularly dissolved is the capacity to orally administer drugs that are difficult to solubilize (Fig. 1).
The choice of an adequate polymer as a matrix to form stable solid solutions is crucial in HME. The polymer must have thermoplastic behavior and be stable at the extrusion temperature. Furthermore, it should have a suitable glass transition temperature between 50 and 180°C, low hygroscopicity and no toxicity, since large amounts of polymer are used. Features such as lipophilicity and a few categories, e.g. hydrogen bonding acceptors (or donors) and amide groups, are commonly prerequisites for a high solubilization capacity. This explains why povidone and copovidone are suitable for HME. In particular copovidone is much more lipophilic than many other water soluble polymers which contain hydroxyl groups and therefore best meets the lipophilicity requirements of poorly soluble drugs. Nevertheless, other entities of different chemistry have been used for HME: polyethylene glycols, polyethylene oxides, hydroxypropyl and ethyl celluloses, acrylates and, most recently, a unique polymer with an amphiphilic chemical structure (Soluplus, Fig. 2).
Pioneer In HME
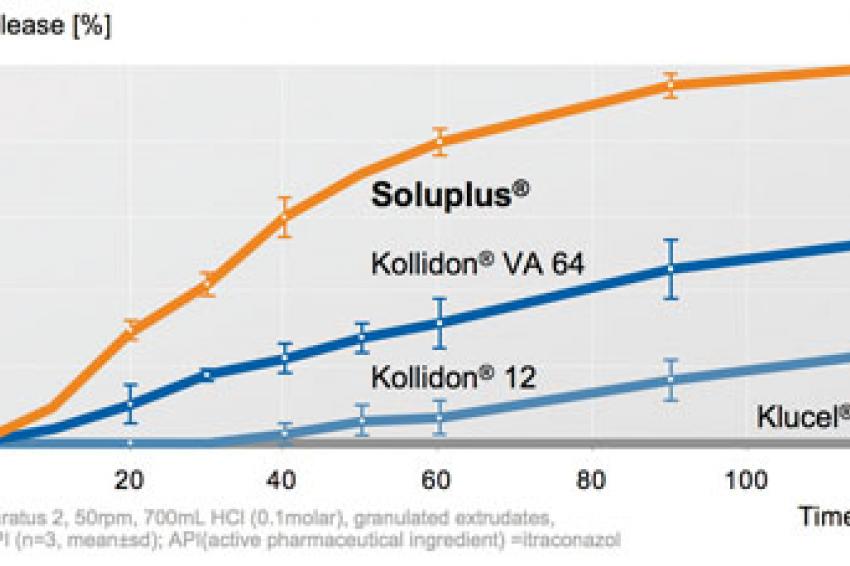
Soluplus is the first excipient in the market especially designed for hot melt extrusion. Dissolution results showed a considerable enhancement in the drug release of poorly soluble active pharmaceutical ingredients, e.g. itraconazole. This study was performed with solid solutions prepared with Soluplus and compared with several polymeric matrices. The best results were achieved with Soluplus (Fig. 3).
Considerable improvements in the oral bioavailability of several poorly soluble drugs, e.g. itraconazole, were achieved with solid solutions prepared with Soluplus. The bioavailability studies were conducted in beagle dogs (Fig. 4).
A wide range of safety studies have been performed on Soluplus and the product safety is demonstrated in comprehensive documentation.
Conclusion
Most of the current drug candidates are barely soluble by regular solubilization methods and therefore not sufficiently absorbed from the gastrointestinal tract. Thus many promising drug products have not been able to reach the market because of failures in bioavailability studies. HME is now opening new doors for drugs that have suffered from this problem.
A detailed list of references can be obtained on request from the author.