Polymers for Fuel Cells and Fuel-Cell Systems
Renewed Interest in Hydrogen Fuel Cells for Long-Range E-Mobility
Electric Avenues - Vehicles with an electric drive train are considered a real option for future mobility. For long-distance driving, many carmakers are reconsidering fuel cells, either as range extenders or as the only power supply. This offers new opportunities for the use of specialized polymers and plastics in the fuel cell itself as well as in the fuel-cell system.
Right Electricity Source for the Right Distance
On the way to "the ultimate eco-car," as Toyota puts it on its website, the public focus in recent years was on hybrids, plug-in hybrids and battery-electric cars. However, a closer look at the websites and presentation of many automotive companies shows renewed interest in hydrogen fuel cells, especially for long-range driving with fast refueling.
For example, BMW CEO Norbert Reithofer has emphasized the strategy to use "the right propulsion for the right distance," and names the hydrogen fuel cell as the option for long-distance driving. Likewise, Daimler and Toyota show graphs on their websites that indicate battery-electric cars for urban driving, hybrids or plug-in hybrids for intermediate distances, and hydrogen fuel cells for an electric drive train that allows long distances. Volkswagen states the same idea on its website.
Toyota announced a range of 700 km for its FCV-R, set to become commercial in 2015, after having reached 830 km with the experimental FCV-adv recently. Hyundai says it has begun a small series production of its fuel-cell car in 2013.
The Fuel Cell
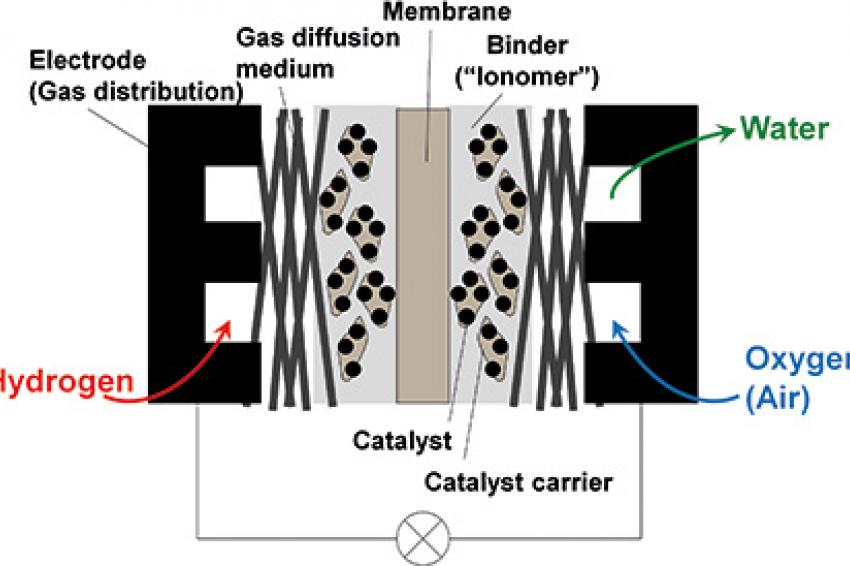
All these developments are aiming at various types of hybrids, from a fuel-cell powered drive train with a relatively small buffer battery, to a battery-powered drive train with a small fuel cell as range extender. Whatever the final commercial solution will be, there are several opportunities for the application of plastics and polymers in fuel-cell systems. Besides the necessity for lightweight construction that is common for all types of vehicles with optimized energy consumption, several components of the fuel cells and the fuel-cell system can be made from polymers. Examples are the electrolyte membrane and the membrane electrode assembly, the bipolar plates (electrode plates) and end plates, gaskets, the periphery - such as air ducts, moisturizer and other components.
The Membrane
A key component typically made from a highly specialized polymer is the electrolyte membrane (Figure 2). It serves as a gas barrier between fuel (hydrogen) at the anode and air at the cathode of the cell as well as an electrical insulator between the electrodes. At the same time, it must transport protons from the anode compartment of the cell to the cathode compartment to complete the controlled oxidation of the fuel by air to form water as the only product of the reaction between hydrogen and the oxygen from air.
For automotive applications, the operating temperature is below the boiling point of water, so liquid water can be present, and the membrane utilizes this water for the proton transport. Under certain conditions the membrane can dry out, which results in a significant loss of its proton transport capacity. In order to avoid this, the water produced by the cell is recovered and used to moisturize the cell. In addition, the membrane polymers must be optimized to require as little water as possible for the proton transport.
There are two major types of polymers used for these membranes: perfluorinated polymers with sulfonic acid groups, the most commonly known of which is Nafion, and hydrocarbon polymers with sulfonic acid groups, mostly based on polyetherketones and polyethersulfones. The acid groups are necessary to maintain a sufficiently high concentration of protons within the membrane to allow fast transport. Yet they also cause a very aggressive environment for the membrane polymer, which must be able to withstand acid hydrolysis at a temperature close to the boiling point of water, oxidation by oxygen at the cathode side, and reduction by hydrogen at the anode side. Only few backbone structures are suitable for this.
The types of polymers mentioned above are durable enough under these conditions. They all combine reasonable mechanical properties, water content, and proton conductivity by forming a microphase separated morphology. This was first discovered for Nafion, where the highly polar sulfonic acid side groups are attached to flexible side chains that can group together, forming polar "domains" dispersed in the nonpolar fluorinated matrix. In the presence of water, these domains are interconnected and form pathways for the proton transport. In hydrocarbon polymers, the sulfonic acid groups are mostly attached directly to the polymer backbone, resulting in a more rigid structure that cannot phase-separate as easily as in Nafion.
However, one can deliberately design a suitable microphase-separated morphology by preparing block copolymers consisting of a long sequence of highly sulfonated repeating units in the center, and long sequences of nonsulfonated units at the ends of each chain. Such materials show high proton conductivity at relatively low water content and good mechanical properties. Optimizing the chemical structure as well as the film casting conditions, the desired morphology can be obtained (Figure 3).
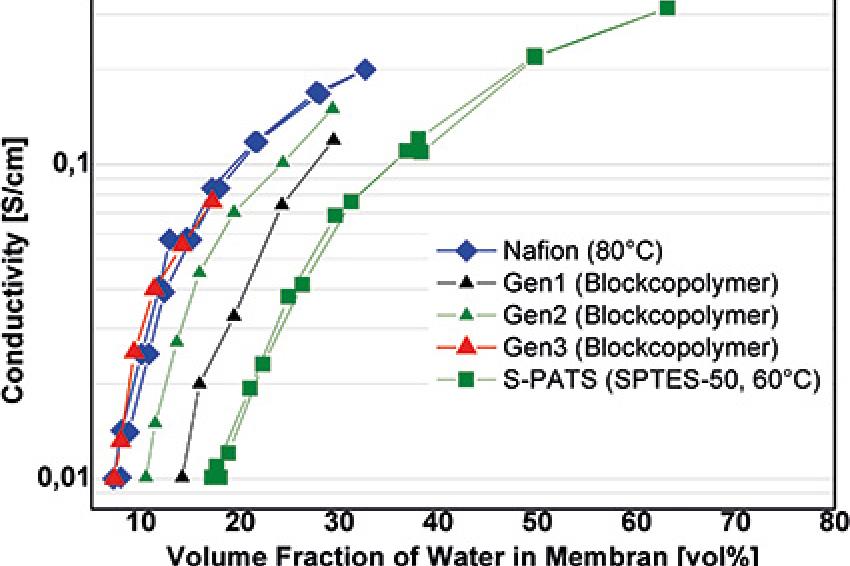
Figure 3 clearly shows that the proton conductivity of the exact same polymer can be increased by a factor of 15, when the morphology is changed from isolated domains to highly oriented domains to a bicontinuous network of conducting and nonconducting domains.
Figure 4a shows the proton conductivity of various polymers in dependence of the water concentration. Perfluorinated Nafion is more efficient (shows a higher conductivity at the same water concentration) than simple sulfonated polyetherketone block copolymers, but when the chemical structure of the block copolymers is optimized by reducing the polarity of the backbone to minimize interaction with water molecules through hydrogen bonds, the same efficiency can be achieved with hydrocarbon polymers.
As a consequence, fuel cells based on such kinds of polymers exhibit constant performance at decreasing humidity down to a certain level. Below that, some performance is lost. Figure 4b shows the performance under reduced humidity of an optimized hydrocarbon membrane with a bicontinuous morphology as shown in Figure 3 and an optimized hydrophobic backbone. The loss of performance under dry conditions is only 15%.
The Periphery
Since the membrane requires acidic groups to maintain a high proton concentration for high proton conductivity, any ionic impurities are a problem. Metal ions from external sources (salt spray in winter) or internal sources (bipolar plates, pipes, valves, humidifier, etc.) could accumulate in the membrane, reducing proton conductivity and, especially in the case of iron impurities, potentially acting as catalysts for decomposition.
Besides stainless steel, plastics are a good choice for all these components, particularly under lightweight construction aspects. In addition to hydrolytic stability, these materials must also not leak ions under the conditions encountered in the fuel-cell systems (typically 95° C, liquid water). Thus, additives and stabilizers, but also residual catalysts, have to be selected carefully.
For bipolar plates, metal, graphite, graphite- or carbon-filled thermosets, and graphite- or carbon-filled thermoplastics can be used. Large fuel-cell stacks need to be cooled to ensure operation in the right temperature range. Coolant ducts can be incorporated into the bipolar plates, resulting in relatively complex geometries: gas channels for hydrogen on the anode side, gas distribution channels for air supply and water removal on the cathode side, and coolant channels inside the plates.
All these items provide opportunities for additional use of polymers and plastics in the automotive industry in the future to pave the way for e-mobility without compromises in range and power.