Innovative Medicine with Rising Productivity Targets
Current Challenges and Potentials for R&D Efficiency in the Pharmaceutical Industry
The need to get products quicker to market, to ensure greater regulatory compliance and to boost efficiencies while reducing operational cost is a challenging goal. Owing to the dynamic market environment, research-based pharmaceutical companies are committing to rising productivity targets.
The causes for rising productivity targets are manifold and related to factors such as patent expirations for blockbuster drugs, increasingly cost-constrained health-care systems or the development of cheaper generic drugs by other market participants. On the other hand, drug R&D costs of bringing a new biological or chemical product to market have significantly increased over the last decade. The increase also applies to R&D timeframes whose delay is due to more complex compliance and regulatory requirements.
The evaluation of the EU R&D Scoreboard 2014 revealed that pharmaceutical companies operating in biotechnology increased R&D by 20.4% (share of R&D investment) whereas the traditional pharmaceutical companies showed a slight decrease of 0.2%, which is mostly due to the fast development of biotechnology.
Innovation Versus Cost Optimization
After a decade of decline, innovation is on the rise for the pharmaceutical industry because of an upturn of approval rates, according to results from KPMG’s Global Life Sciences & Development Survey in 2013. Innovation is the major strategic success driver, which has to be accompanied by a rapid market launch of the new product for generating profit. Nevertheless, ambitious productivity targets, which are often supplemented by cost optimization programs, have to be met. An exploitation of cost-cutting potential, however, may have a significant limiting or negative effect on companies’ innovative strength if not carried out specifically. Adverse effects usually arise if cost reduction initiatives lead to the delay of programs, a lack of empowerment, delegation upward or frustrated scientific experts.
Smart Compliance Versus Bureaucracy
One of the main challenges regarding higher productivity is managing risk without restricting innovation. Hence, successfully achieving productivity targets means mitigating the risks that are connected to medical innovation processes. The pharmaceutical industry is faced with a higher-than-average level of R&D project risks, which is reflected by high attrition rates.
At the same time, pharmaceutical companies operate in one of the most heavily regulated industries and have to cope with high compliance and regulatory standards. As the price of failure places more and more at risk, there is a strong motivation to make more conservative research that may restrain the acceleration of drug discoveries. Similar reasons may also lead to additional testing on active ingredients with lower benefits. Both of these motives result in an increase of R&D costs as well as in an extension of the R&D timeframe, which is directly followed by a delay in market launch. Being only the second on the market behind a competitor can have severe consequences regarding painful profitability losses and a jeopardy of return on R&D investments.
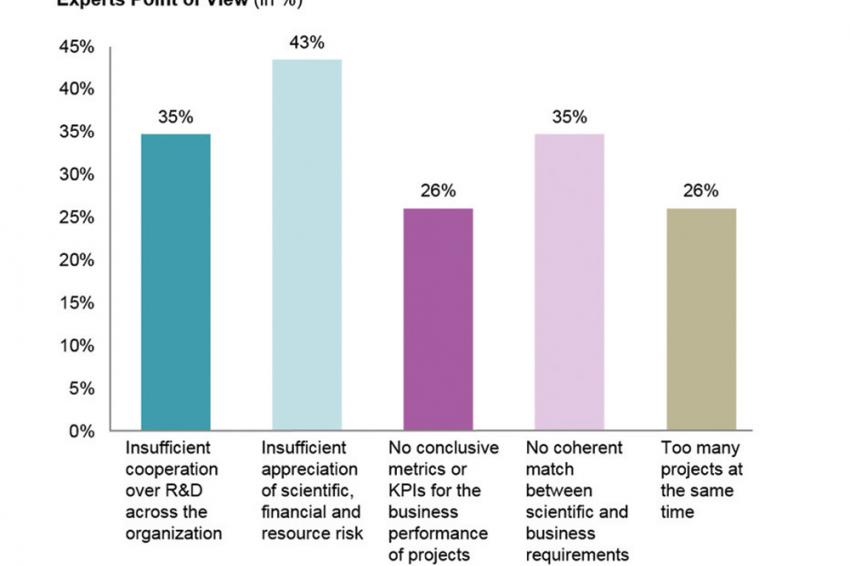
Apart from balancing smart risk-taking and fulfilling complex compliance as well as drug safety and efficacy requirements, a missing coherence of scientific and business requirements is considered as another main challenge to productivity for R&D executives (compare fig. 1).
Barriers For Innovation
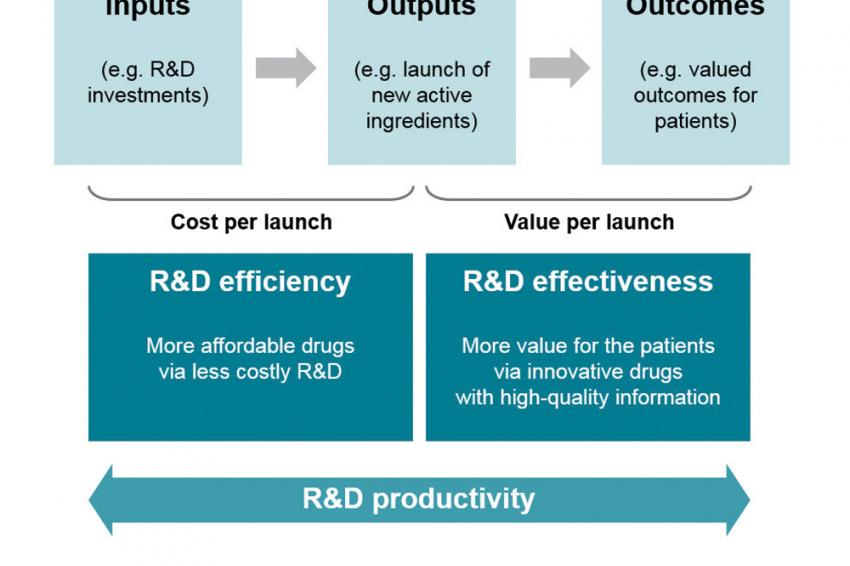
For enhancing R&D productivity within the drug development process, it is crucial to understand the underlying interfaces between inputs, outputs and outcomes (compare fig. 2). R&D efficiency outlines the ability of an R&D organization to transfer inputs (e.g., ideas, R&D investments or research effort) into tangible outputs (e.g., new active ingredient launches), whereas R&D effectiveness can be represented as the ability to provide outputs with certain intended and desired qualities (e.g., medical value to patients or physicians as well as a profound financial outcome). R&D productivity encompasses both R&D efficiency and R&D effectiveness.
For executives the main barriers for a successful improvement of R&D productivity are a lack of business training for R&D leaders, insufficient information flows between project organization and cost centers, an inadequate involvement of other neighboring functions and in particular excessive administrative work (non-project time) for scientists, which is hindering R&D potential. The presence of non-standardized processes as well as complicated approval and decision processes are considered to be further obstacles for a leaner drug development process.
Untapped Opportunities for R&D Productivity Enhancement
Considering what is needed for improving R&D productivity — several unexploited potentials in pharmaceutical companies should be examined. A large part of improvement potential still lies in complexity reduction of processes and interfaces. Some companies get stuck between cost-cutting initiatives on the one hand and the ramp-up for product launches on the other hand, which leads to frustration of experts and co-workers who have to implement both initiatives.
Overarching and unspecified percentage-cuts can work in opposite directions as it bears a high risk of disrupting the R&D flow and reducing the company value. For instance, inadequately aligned outsourcing initiatives have jeopardized data quality and cut through trusted and established networks.
Based on the fundamental premise that scientists should focus on science, it has to be secured that innovative research is not confronted with bureaucratic obligations, so that non-medical tasks (e.g., scheduling, resource management, administrational oversight) are in a right balance with the risk and complexity of research or clinical projects.
Admin and budgeting processes should be improved or centralized where needed and removed where possible. An activity-split-analysis helps to identify tasks with an administrative or transactional character that can be moved out from the processes/responsibilities of medical experts to a center of excellence with appropriate skills and economies of scope without disrupting the R&D flow. The positive result is more focus on customer and patient needs. The establishment of a medical center of excellence can reduce the workload of medical experts regarding project management; for example, a dispatcher coordinates the schedule and necessary interaction during the development and implementation of a study so that all participating functions are optimally aligned.
For an enhancement of R&D productivity the unspecific roll-out of standard operating procedure (SOP) trainings has to be changed to an SOP approach at the right time (when needed), with the adequate content (what is ahead in the next time period) and for the right medical experts (who in the department and who within a certain role description). The benefit is that SOPs take on real meaning for the reduction of risk instead of being treated only with half attention by clicking through the SOP training program.
Unnecessarily precise requirements regarding the business planning and budgeting of studies should be challenged in two ways. First, bottom-up planning without an appropriate guidance can lead to frustration of the involved medical experts because after a first budget proposal is created, top-down changes of senior management render the first draft obsolete and require a complete rework of the budget. Second, based on the effect that in early studies, the attrition rate is significant, a lot of work that goes into backups of business plans and approval templates can be saved through a lean approach whereas phase 2 and phase 3 studies should be planned with increasing details and requirements for precision as part of a smart risk-taking approach.
Figure 3 gives an overview of some potential strategies for boosting R&D productivity.
Nevertheless, the achievement of productivity targets strongly depends on further organizational key success factors. A coordinated approach with financial control of R&D productivity improvement is needed. Finance and Controlling should be involved from the beginning and maintain the overview of business cases and financial target setting. Change management has to be integrated while processes are simplified so that people experience quickly the added value of new structures.
Contact
KPMG
Am Flughafen - The SQUAIRE
60549 Frankfurt
Germany