Recombinant Human Albumin
A case study in excipient quality and functionality
Modern Excipients - Traditionally, excipients have never received the same attention as that given to the Active Pharmaceutical ingredient (API). Instead they have been seen as "inert" materials that confer a suitable form or consistency to a formulation to facilitate delivery. Increasingly, the perception of excipients is changing and these materials are now seen as functional components of a pharmaceutical formulation, where they contribute significantly to both the functionality and stability of the final product.
The change in the perception of excipients and the need to implement a "quality by design" approach to drug manufacture means there has never before been a greater need to understand the critical quality attributes of an excipient product. Furthermore, it is crucial to understand how these quality attributes relate to performance in the final formulation and acceptability to the regulatory authorities.
Typically an excipient material will be available from multiple suppliers and a drug manufacturer will have to select the most appropriate material for their purposes based on the quality and functionality requirements. Often the initial selection of materials for evaluation will be based around examining the specifications and certificate of analyses. These may often appear comparable between suppliers and may not reveal distinct differences that could dramatically affect the acceptability of the materials to the drug manufacturer.
The needs of the pharmaceutical industry are recognized and special recombinant albumin products, Novozymes Recombumin and Albucult have been designed to meet the highest quality standards. There are a number of commercial products that are intended for use in this market. In this article, some of the key quality attributes of Novozymes products in comparison to other commercially available recombinant albumins are compared. Some of the key challenges around selecting an excipient material that is fit for purpose are highlighted.
Commercial recombinant albumins
Plasma derived human serum albumin has a long history of use as a stabilizing agent for vaccine and protein formulations. However, concerns over the potential for transmission of viruses and transmissible spongiform encephalopathy from pooled plasma and proteins derived from it led to adoption of recombinants albumin products. The world's first and second recombinant human albumin products, derived from Saccharomyces cerevisiae are manufactured by Novozymes Biopharma. Today a further 3 recombinant albumin products are available and a summary of these is presented in Table 1. All of the products are purportedly indicated for biopharmaceutical use and have a purity of claim of >95% or greater.
GMP certification
A first point of interest when selecting an excipient material may be the origin and compliance with good manufacturing practice (GMP) guidelines. Whist nearly all components of a final drug product are required to be manufactured to GMP guidelines; no such global regulatory requirement exits for excipients2. Work in this area began in 2008 when the FDA acknowledged that GMP certification for excipient products may be beneficial to the pharmaceutical industry.
This work was taken further by the International Pharmaceutical Excipient Auditing (IPEA) program that worked to define these standards in 2009. Whilst this GMP certification is not mandatory and is not a direct indication of final product functionality, it does have some advantages in reducing the burden of auditing an excipient manufacturer. A lack of compliance to GMP may be enough for some manufactures to rule out an excipient material. When considering the commercial recombinant albumins only 3 of the supplies have products reported to be in compliance with GMP guidelines (Table 1).
Product purity and homogeneity
Purity is often a key driver in the selection of an excipient. Understanding the purity of a protein excipient such as a recombinant albumin is complex. In addition to understanding the presence of other components in the final formulation, such as host cell protein levels, attention must also be given to the homogeneity of the protein itself. The presence of modified forms of the protein, in significant quantities, such as glycosylated or partially degraded protein can have a significant effect on the functionality of the protein, as well as decreasing the acceptability of the material to regulatory authorities.
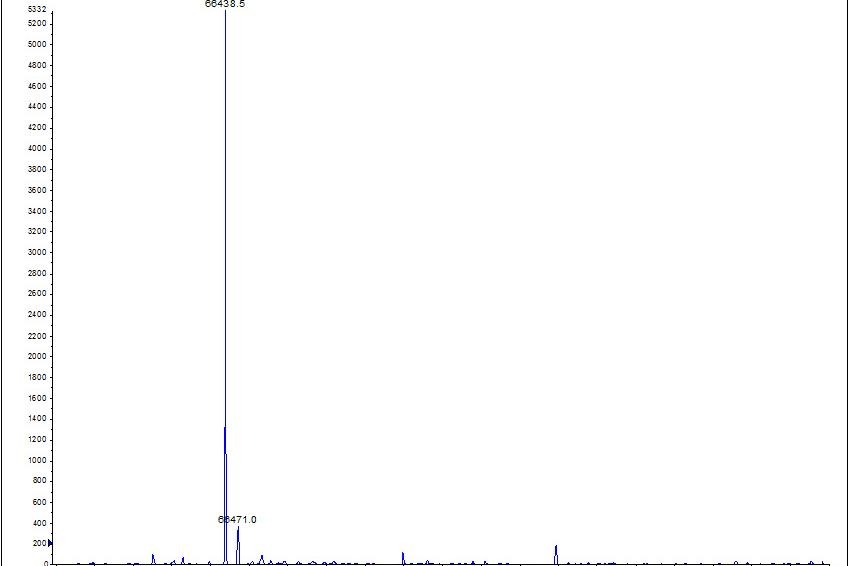
All of the commercial recombinant albumin products described in Table 1, have a protein purity specification of 95-99% based on gel electrophoresis. While this is a standard technique for the determination of this parameter, it may not fully resolve the heterogeneity of the protein. We have performed an analysis of these products using conventional gel electrophoresis (Figure 1) and a more detailed evaluation using mass spectrometry (Figure 2).
The gel electrophoresis data for all the products evaluated demonstrate a single band at ~64 kDa that is representative of the native intact albumin molecule. The exception is commercial albumin 1 that has distinctive bands at a lower molecular weight than the native albumin molecule.
Despite the relatively similar appearance between the products by gel electrophoresis, the mass spectrometry analysis reveals a more interesting picture (Figure 2). Recombumin and Albucult both demonstrated a single species at 66.4 kDa that is representative of the expected native human serum albumin molecule. In comparison, all of the other commercial products contained multiple peaks that were indicative of partially degraded or glycosylated forms of the protein in the final formulation. Perhaps the most striking mass spectrum was recorded for the rice-derived product from Supplier 1. This product displayed ~40 peaks in the mass range evaluated. On the basis of this analysis it is clear that only a small proportion of the protein present is native human serum albumin. This is despite the labeled purity claim of >96% (w/w).
A further evaluation of purity can be made by visual inspection of the products. This type of inspection is often one of the first analytical tests performed to examine product color and clarity. This parameter is important since it is often considered a direct indication of product quality and purity. In instances where the material is likely to be present in relatively large quantities, such as in a final pharmaceutical formulation, a significant increase in product pigmentation due to the excipient may be undesirable.
The images presented in Figure 3, demonstrated that Recombumin and Albucult were the least pigmented of the products evaluated; with both products having a clear/straw color. The alternative products showed increasing levels of pigmentation with the albumin from Supplier 1, a rice-derived product, showing the greatest pigmentation and the final product having an orange/amber color.
Functional Characterization
Many of the physicochemical properties of an excipient product can influence the performance in a formulation. In many instances, some of these characteristics may be application-specific and may require characterization that is beyond that contained on a typical C of A. Where this is required it may be necessary for the drug manufacture to work with the excipient supplier and to identify such properties and device appropriate testing procedures.
An example of this type of functional characterization in relation to recombinant albumin molecule is in the area of chemical conjugation to albumin for peptide or protein half-life extension. The albumin molecule contains a natural free-thiol group that is a natural antioxidant for stabilization but is also used as a specific target in many chemical conjugation reactions. Whilst this group is intrinsic to the native albumin molecule, it can become oxidized and deactivated due to poor storage or processing. Low levels of free-thiol in a conjugation context may lead to poor product yields that could ultimately increase manufacturing costs. In this context, it is important to work with a recombinant albumin supplier that understands the importance of this parameter and more importantly, how to control it.
The free-thiol content of the commercial rAlbumins already described in this note were evaluated and the data is presented in Table 2. It is obvious that free-thiol levels vary between products, with Recombumin and Albucult having the highest levels of all the albumins tested. Commercial albumin 3, derived from rice, is almost completely blocked and would not be suitable for conjugation applications through this chemical group.
Summary
As the perception of the role of excipients continues to change, the level of scrutiny given to the quality aspects of the products increases. Some of the key quality and functional features of a range of commercially available recombinant albumin products were evaluated. It is clear that there is distinct variability in the quality attributes between the suppliers of these products, despite potentially appearing quite comparable to the end user. In particular, the differences between the materials in terms of compliance of the manufacturing process (GMP), protein purity and protein homogeneity have been identified.
All of these factors may reflect in the acceptability of a material to a pharmaceutical company. The potential for this variability between suppliers of excipients highlights the need for the excipient manufacturer and drug companies to work in partnership to understand the properties of the excipient material in terms of quality, origin and performance. Whilst the focus of this article was recombinant albumins, it is likely that similar scenarios will exist for numerous other types of excipient materials.
Contact
Novozymes Biopharma UK Ltd.
59 Castle Blvd.
Nottingham NG7 1FD
+44/115/9553355
+44115/9551299