Quality and Efficiency in Pharma Manufacturing
Process Analytical Technology as a Key to Quality by Design
The pharmaceutical industry is confronting a variety of new and complex challenges in the face of globalization and changing market dynamics. The past decade has witnessed a decline in the discovery, approval and marketing of new chemical entities with fewer blockbuster drugs making it to the market, as well as stiffer competition from generics. Stricter regulatory requirements demand continuous investment to track products and document processes.
The pharma industry stands out among manufacturing industries for the astronomical amounts it spends every year on research and development – a stark contrast to the relatively low-tech level of manufacturing.
Pharmaceutical manufacturers are addressing the challenges of manufacturing efficiency with stronger cost-containment measures and initiatives to improve operational excellence and quality. In North America, the US Food and Drug Administration (FDA) has championed one such initiative, known as Quality by Design (QbD), with the goal of improving productivity and reducing manufacturing costs for US producers. This initiative is part of a larger effort to reduce overall health-care costs based on the assumption that drug costs are high because of inefficient manufacturing.
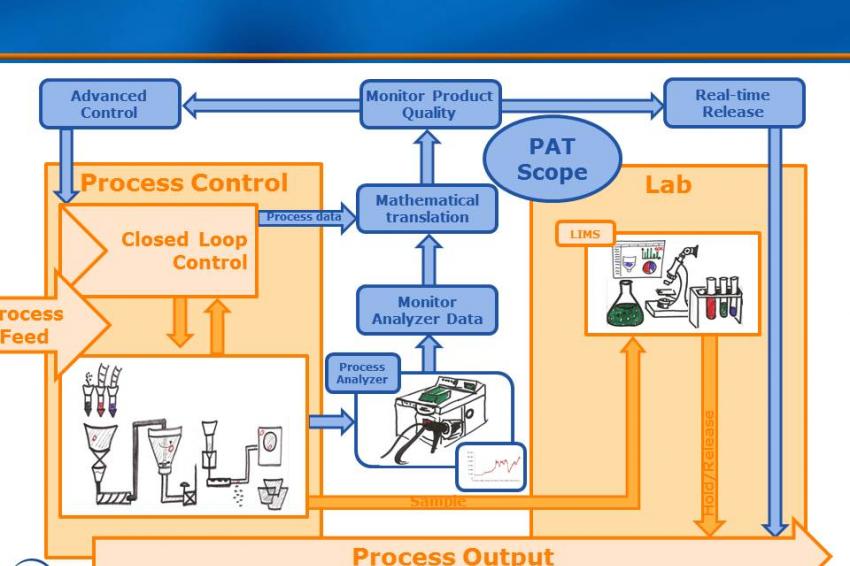
Process analytical technology (PAT) is an enabling technology for QbD. PAT tools enable in-line measurement of critical quality attributes and process parameters in real time, allowing running processes to be adjusted on the fly. Product quality is verified in multiple steps during process execution rather than performing QC at the end of the line, helping to shorten batch-release times. In addition, correcting the course of a batch that is threatening to go out of spec can dramatically improve yield. The business benefits of PAT tools are promising: They include more efficient use of equipment, higher batch yields, less waste, shorter time-to-market, and significantly better process understanding and control.
The Problem of Quality by Testing
Quality by testing is the traditional approach to quality control that compares finished drug products to an approved specification and rejects anything that doesn’t meet it. Quality is assured by testing materials before and after manufacturing, all within a strict, fixed process. This process must be tightly controlled because of the uncertainty of whether the specification alone is adequate to ensure quality. The downside is that overly stringent specifications may result in unnecessary recalls or drug shortages.
The inherent problem in this approach is that root causes of failure are poorly understood, and as a result, manufacturers risk ongoing, unexplained losses. This can continue indefinitely or until supplements are filed with a government authority to allow broader acceptance criteria. But changing validated processes is prohibitively expensive, so quality is maintained through rigid manufacturing practices and by testing end products, without considering how system and process design can ensure product quality.
This method also creates problems during the move from laboratory pilot to full-scale production. Production specifications are typically derived from test data from small batches in the laboratory environment. Scaling up to commercial production can reveal new complexities that were not evident during development. As production levels are reached, the regulatory requirement of filing supplements for minor and incremental changes to manufacturing processes makes it difficult to improve the process with closed loop feedback to achieve continuous, real-time quality assurance.
Quality by Design
One of the most talked about movements in QA in recent decades is Quality by Design (QbD), a methodology that assumes quality can be designed into a product. According to QbD, most quality problems are a result of the way quality was planned (or not planned) in the first place. Instead of raw material and end-of-line testing, QbD focuses on designing and developing manufacturing processes to ensure that predefined product quality requirements are met. This built-in quality originates with the careful design and control of these processes. The result is a well-understood product that consistently meets quality specs.
PAT refers to the measurement and evaluation tools that enable QbD. PAT tools consist of in-line sensors and analyzers (spectroscopy instruments, e. g., NIR or near-infrared) that measure substance properties during the manufacturing process and pass on these data in real time for evaluation. Software tools translate these data into product quality information (the CQAs) that infer final quality of the batch and can be used to adjust the running process to ensure that targeted quality goals are achieved. Tools for advanced process control (APC) play a role here.
In support of QbD, PAT’s goal is to ensure final product quality by designing, analyzing and controlling process variability through timely measurements of critical quality and performance attributes of raw and in-process materials. This applies not only to manufacturing processes, but also to development. With in-line analytics, product developers can gain a much higher and faster understanding of the process than is possible with offline analysis (manual sampling). The real-time access to information offered by PAT is invaluable during pharmaceutical development and during the scale-up to commercial production.
PAT in Product Development
Within the QbD framework, PAT tools can be applied as early as the development phase of a new product. Once an active ingredient has been developed in the laboratory, the next step is to decide on dosage and delivery (tablet, capsule, suppository), and which excipients to use. These factors then determine the processes used to manufacture the drug product.
During development, in-line analytics allow faster and clearer understanding of process dynamics versus traditional offline analysis. Performance requirements at this stage will differ significantly from those in the later manufacturing process, but the parallel application of these tools in both areas allows a better understanding of the specific challenges of the process. It also facilitates scale-up to production levels thanks to a deeper process understanding.
PAT in Process Design
PAT is also an integral part of manufacturing process design. The use of PAT tools enables users to develop robust, well-understood processes with defined parameters that allow for real-time monitoring and adjustment of CQAs. During process development, raw materials, process parameters and CQAs are investigated to determine raw material attributes, process parameters and CQAs for each process, and to identify relationships among them. In the scale-up to commercial manufacturing, parallels to the development phase can be exploited to shorten process development and time-to-market. Knowledge is not static – rather it builds throughout the manufacturing life cycle, and the deployment of PAT tools along this cycle supports this notion.
Real-Time Release Testing
In the pharmaceutical industry, strict regulations and regular quality checks can lead to frequent, costly delays and unnecessary scrapping of materials. This creates inefficiencies that lower overall productivity. The idea of measuring product quality in real time with in-line analyzers has been talked about for a decade, but implementation is still relatively new, despite compelling business benefits.
Real-time release testing (RTRT) is defined as the ability to ensure product quality during the process based on real-time process data instead of end-of-batch QC. RTRT uses PAT tools to gather data from analyzers and other inline process measurements to generate quality information that can be used to adjust the process during batch execution. While it doesn’t replace the review and quality control steps required by good manufacturing practice for batch release, RTRT can eliminate end-product quality testing, cutting out delays and streamlining the whole manufacturing process. A side benefit is that the integrated quality information automatically becomes part of the batch record.
PAT in Continuous Processes
Manufacturers often employ batch-control methods with batch sizes determined by production schedules, capacity, or specific customer orders. While continuous processing is known to have distinct advantages over batch, quality-control requirements force pharma manufacturers to think in terms of finite lot sizes. However, that mentality is changing – thanks in part to the availability of PAT tools.
By providing continuous, real-time quality information, PAT essentially turns a batch process into a continuous process, allowing constant quality checks along the way that eliminate the need for a final quality check to release a batch. Instead of producing finite quantities, manufacturers can “stream in” and “stream out” enough raw materials and finished products to meet demand. The advantages of a continuous process lie in the more efficient use of equipment, higher productivity and better quality.
Quality by Design takes a different approach to ensure consistent levels of quality by allowing for flexibility during the manufacturing process. PAT tools play a crucial role in the implementation of QbD by providing the real-time feedback that allows operators to keep CQAs in spec by adjusting processes during operation. At the same time, quality data collected during the process can take the place of end-of-line QC, drastically cutting batch release times. The many business benefits of employing PAT tools are highly attractive.
PAT Can Turn Batch Processes into Continuous Process
The most important aspect of a PAT implementation is to retrain a company’s culture to approach quality with a proactive rather than reactive mindset. To achieve this, cross-functional, multidisciplinary teams are necessary to effect change management to devise and implement the new processes that take full advantage of PAT. A successful PAT implementation requires a rethinking and a reorganization of resources. Users should anticipate an increased need for statistical analysis and more control engineering skills as analyzers are integrated into production systems. A key success factor, however, is to bring together development and production teams at the earliest stage to ensure the buy-in of top managers of these departments.
On the technical side, it’s important to understand that PAT tools and devices won’t simply automate existing processes. Instead, processes like manual quality checks are replaced by automated measurements. This information in real time allows the process to be adjusted, but this new flexibility must be built into the process control strategy – a challenge that will require program changes on existing lines, or consume development resources when engineering new lines. In addition, production equipment should have redundant systems in place in the event PAT equipment should fail.
Costs and Risks of Not Investing in PAT
Making a business case for PAT is not straightforward because it is difficult to estimate future returns from improved quality or the lower costs of generating less waste. A PAT investment is not just in equipment, but also in people and processes, so those costs must be amortized over a longer payback period. ARC recommends taking a holistic view to the PAT value proposition that not only considers the initial capital outlay, but also weighs the costs of organizational change against the long-term benefits.
The pharmaceutical industry is known for high-tech research and development but relatively low-tech manufacturing. PAT technology is changing that. In the past, the heavy R&D focus and strict, validated processes made it nearly impossible to improve manufacturing processes with optimization tools commonly used in other industries. Started by the U.S. Food and Drug Administration to proactively improve the productivity of American drugmakers, the PAT initiative has become a chance for all manufacturers to add sophisticated technology to their process control with the cooperation of government authorities, rather than being held back by regulations. As the industry adopts PAT, pharmaceutical manufacturers may not be able to afford not to invest in PAT due to the risk of losing competitiveness.
Making a Batch Process Continuous with PAT
Faced with the pressures of globalization, some pharmaceutical manufacturers are answering their challenges with innovation. One of the top five global pharmaceutical manufacturers recently employed Sipat in a pilot project. Instead of just designing PAT tools into the tablet-making process, the company took the bold step of converting a classic batch process into a continuous process using Sipat. The goal was to take an innovative approach to improve some of the chronic inefficiencies inherent in this type of process, including high inventory requirements, long changeover times, disconnected processes, high process losses and low asset utilization.
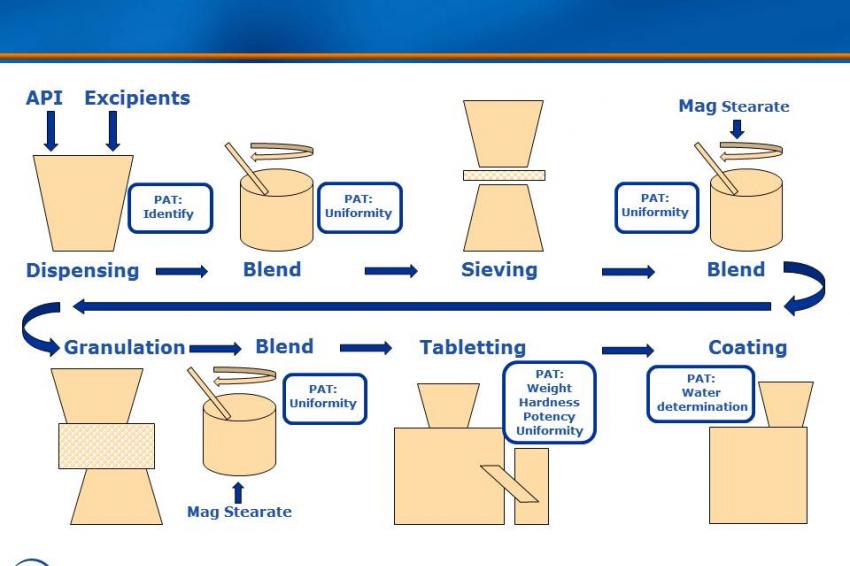
The production line applies high-shear wet granulation process technology to produce oral solid dosages (tablets). The line consists of unit operations for granulation, drying, milling, blending and compression, and is controlled by two PLCs (SIMATIC S7-300) with WinCC for line visualization. Near-infrared (NIR) analyzers measure properties such as moisture, content uniformity and assay, and laser diffraction is employed for particle-size detection.
In this application, Sipat software collects and evaluates multivariate product quality information (CQAs), such as loss on drying or particle-size distribution, as well as univariate process data such as speed, torque, temperature and compression forces for closed-loop model predictive control. Quality parameters are shared with a manufacturing execution system (SIMATIC IT) that tracks this information for real-time release reporting. Sipat allows parameters such as moisture content, content uniformity, hardness and thickness to be adjusted should a batch start to veer out of spec, thus maintaining quality in real time and greatly reducing the risk of having to scrap a whole batch at the end of the process. By using PAT tools, company engineers were able to deepen their process understanding much faster. The scale of the process equipment used for continuous manufacturing in the development phase is the same as for commercial production, so the company was able to eliminate the scaling-up process.
According to the manufacturer, the new continuous process concept was developed within two weeks and the line was producing tablets reliably and with excellent quality after just six months. The in-line quality checks now make possible real-time release with significant increase in efficiency versus traditional batch methods with end-of-line QC. Interestingly, the equipment has a substantially smaller footprint – requiring only about a tenth of the space needed by an equivalent batch line. The reason is that a batch process requires all raw materials to be stored locally during the batch process. A continuous process, on the other hand, employs a continuous flow of materials that don’t need to be stored at a single location. The result is smaller requirements for clean room space, lower clean room HVAC energy usage, fewer operators, and less work-in-process.
Hot Melt Extrusion as a Continuous Process at MSD
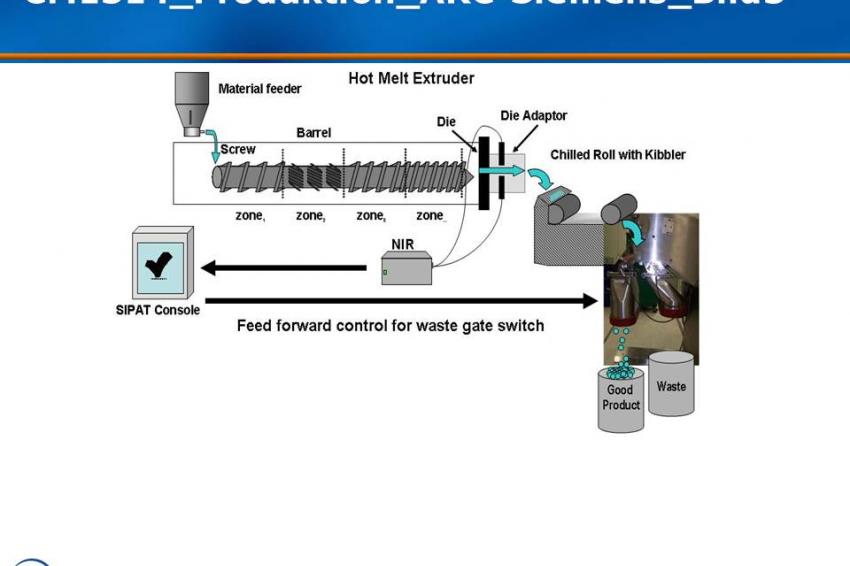
Extrusion is a process commonly used to manufacture plastic parts by forcing molten material through a die under pressure. Merck Sharp & Dohme Corp. (MSD), a subsidiary of Merck & Co., employs a hot-melt extruder (HME) in a process for manufacturing solid oral dosages. This process allows a drug substance to be stabilized in its amorphous form within a polymer matrix, increasing solubility and in vivo exposure, which is useful when the active pharmaceutical ingredient has low solubility. The HME process combines multiple steps such as mixing, melting, degassing and densification, which increases efficiency by reducing the number of unit operations. MSD employs PAT tools and Sipat software from Siemens, using Fourier-transform near-infrared (FT-NIR) spectroscopy to monitor form change, assay and uniformity of the mixed substance.
Extrusion is essentially a mixing process during which process parameters for temperature, mixing and feed rate can be adjusted. Extrusion occurs when the mixture is forced under pressure through an orifice in the die. As the extrudate leaves the die, the molten substance drops between two chilled rolls, causing its temperature to fall below its glass-transition temperature and form a brittle glass sheet. The sheet is then broken into smaller flakes of glass to make it easier to mill. In the final steps, the material is milled, blended with excipient, and then lubricated and compressed into a conventional solid oral dosage form.
The extruder’s screw and barrel are modular, allowing flexible control of venting, material feed, mixing, heating and cooling. PAT tools (NIR, Raman, ultrasound or UV-VIS spectroscopy) come into play at the moment of extrusion. These devices interface to Sipat software from Siemens, allowing CQA deviations (measured here as percent drug loading [wt/wt]) to be identified in real time. If drug-loading predictions are outside of specification, alarms trigger, immediately activating a switch gate to divert out-of-spec material to waste. Any CQA deviations are brought back into line by adjusting the necessary process parameters.
According to MSD, the HME platform can be easily scaled up to production levels or scale-up can be eliminated, depending upon product volume forecasts, because the extruder used for most of process development is essentially the same size as the one used later in production. If scale-up is required, CQAs remain the same while process parameters are scaled up to manufacture large batches of extrudate – up to several thousand kilograms. After the successful development and scale-up of a pilot project, MSD has now deployed the HME platform to additional sites.
Last Word
Quality by Design takes a different approach to ensure consistent levels of quality by allowing for flexibility during the manufacturing process. PAT tools play a crucial role in the implementation of QbD by providing the real-time feedback that allows operators to keep CQAs in spec by adjusting processes during operation. At the same time, quality data collected during the process can take the place of end-of-line QC, drastically cutting batch release times. The many business benefits of employing PAT tools are highly attractive.
Contact
ARC Advisory Group GmbH & Co KG
Stadttor 1
40219 Düsseldorf
Germany
+49 2104 542 012