Understanding the Requirements of Safe HPAPI Manufacturing
Growing HPAPI Demand and Project Complexity Make CMO Partners Critical for Success
For many pharmacologically active substances behind these products, biologic activity is exhibited at extremely low concentrations. Such highly potent active pharmaceutical ingredients (HPAPIs) now account for around one quarter of all new pharmaceutical entities, and half of those in clinical development.
A typical HPAPI has a therapeutic daily dose of less than 10 mg and an occupational exposure limit (OEL) below 10 μg/m3 of air as an eight-hour time-weighted average (TWA). However, a growing number of pharmaceutical compounds under development, known as ultra-HPAPIs, can require even more extreme levels of exposure control down to an OEL range of 1-20 ng/m3. Most HPAPIs and ultra-HPAPIs in clinical development are outsourced to contract manufacturing organizations (CMOs) that have the necessary process expertise, qualified facilities, development labs and track record for compliance with key quality, regulatory and safety requirements. While many CMOs have made significant investments in their HPAPI capabilities over the last two decades, pharmaceutical companies can still find it challenging to select a partner to reliably support them throughout all project stages including process development, clinical scale-up and commercial manufacturing
.
Complex Processes Require Substantial Expertise
Only a few CMOs have a long history of execution in optimizing safety and containment control processes to handle and manufacture HPAPIs from small-scale development lab quantities up to large-scale commercial volumes. The ability to demonstrate extensive project experience with other comparable HPAPI projects, and a strong track record for audits and supply security, is also considered essential.
Furthermore, many HPAPIs require between several and a dozen or more process steps for synthesis. For many of these steps, multiple advanced technologies can be required including peptide coupling, mPEG/PEGylation, continuous processing, chiral resolution, catalysis or biocatalysis and cryogenic reactions. In many instances, only a few CMOs will have the necessary technology portfolio to coordinate the safety and special process engineering capabilities required for such complex projects.
Most importantly, the CMO must be able to demonstrate strong technical competencies in the development of effective risk control systems that are based upon a comprehensive assessment of exposure hazards and other safety risks for each process step required for chemical synthesis. Various disciplines must be aligned together under a HPAPI project team including process engineers, R&D and manufacturing experts, toxiologists, industrial hygiene specialists, and safety and hazard assessment experts. Specialist analysts are also required to implement in-process, intermediate and final HPAPI analyses, and to develop trace analytical methods to support plant cleaning protocols. Additional resources and software for project management, employee train-ing and cleaning protocols should also be available. Often, the CMO will lack the desired technical experience or structural versatility to provide peace-of-mind during the development and implementation of a risk control system.
Key parties and processes that should be utilized under a best-in-class system for hazard assessment, risk assessment and risk control are summarized in the table.
It is a key regulatory requirement that prospective hazards which may be encountered during HPAPI handling are identified and understood to enable OEL development. This highly complex process requires extensive technical know-how. Together with occupational exposure banding (OEB) and other factors including short-term exposure limits (STELs), the development of an OEL enables organizations to conduct assessments for various acute, allergenic, corrosive, carcinogenic, mutagenic, toxic, reproductive, and other occupational risks. These assessments in turn help establish and maintain safety and containment control procedures that minimize exposure risk regardless of manufacturing stage or production site.
Thorough Consideration Quality and Safety Procedures
Conformance to regulatory requirements and ICH guidelines also requires a thorough consideration of quality and safety procedures to prevent either the release of an HPAPI or intermediate, or cross-contamination with other products manufactured within a multi-purpose facility.
“It is a key regulatory requirement that prospective
azards are identified and understood.”
In order to optimize the risk analysis strategy for HPAPIs, Evonik utilizes a system of five Occupational Exposure Bands (OEBs) that incorporates global containment guidelines for its manufacturing sites (cf. figure).
The compound’s OEL classification is based on toxicology data, pharmacology, bioavailability, clinical trial results, and physicochemical evaluations. In-house toxicologists will refer to the data set provided by a pharma customer and also conduct an extensive evaluation of available literature. The most relevant pharmacological and toxicological data set linked to the most serious health effect is selected as the point of departure (POD) for the acceptable daily exposure (ADE) and OEL calculation. This POD value is then adapted applying different standardized toxicology safety factors to calculate the ADE used to determine cleaning requirements of pharmaceutical reactors and equipment, and the OEL (in µg/m3 or ng/m3) to assess containment measures regarding inhalation exposure.
The occupational banding system provides an initial guideline to help the CMO select the most suitable facility and design the containment of unit operations. This is followed by thorough task-by-task exposure risk assessments of the whole drug substance handling process to evaluate specific exposure risks based on the established OEL, as well as pharmacological and toxicological actions of the compound, to protect the operators and prevent potential cross-contamination.
Each process step requires significant attention to the development of safe handling and containment system protocols that can prevent workforce exposure, accidental release of the substance or another pharmaceutically active intermediate, and cross-contamination with other products manufactured in the facility. To facilitate the efficient design of containment solutions in pharmaceutical plants, collaboration with external equipment vendors and engineers is also becoming a key CMO requirement.
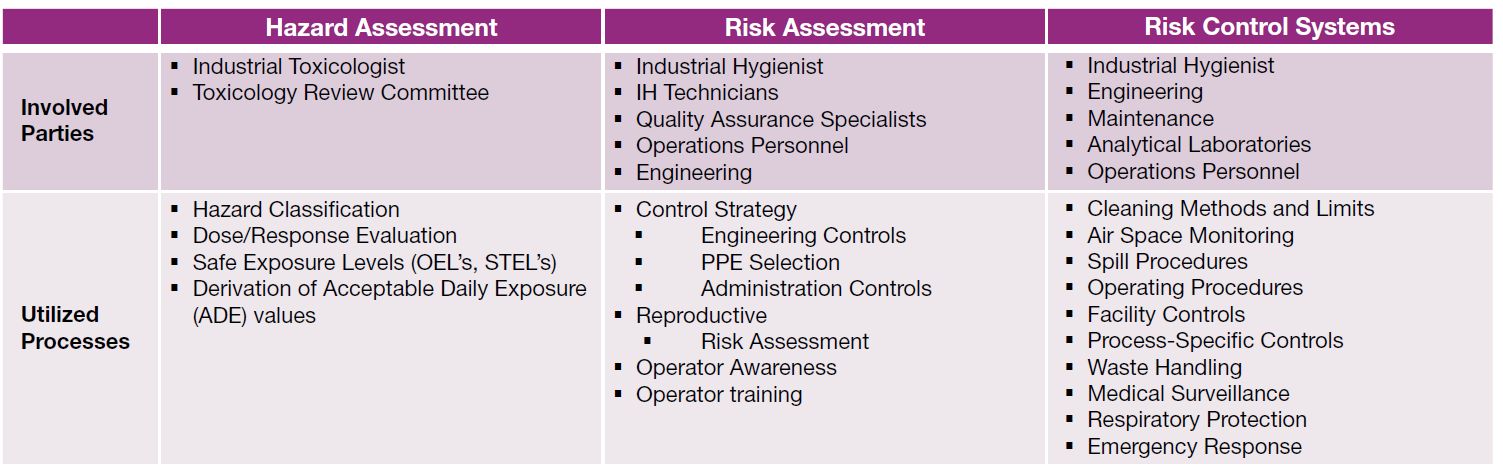 Table: Key parties and processes that should be utilized under a best-in-class system for hazard assessment, risk assessment and risk control.
Table: Key parties and processes that should be utilized under a best-in-class system for hazard assessment, risk assessment and risk control.
Best-in-class Policies for HPAPI Handling
Taking these and other factors into account, Evonik has implemented what it considers to be a set of best-in-class policies for the hazard and risk assessment of highly potent compound handling:
- Toxicological assessment of compounds with pharmacological and/or toxicological activity and setting of OEL and ADE values. In a case where data is not sufficient to derive an OEL, a basic hazard review is conducted to assign a default OEB range. The application of estimated in-silico predictions of mutagenicity using FDA-accepted software tools is provided.
- Industrial hygiene risk assessments prior to handling HPAPIs, and an ongoing program to monitor the performance of containment systems.
- Guidelines for facility design and exposure control approaches to establish the safe handling of potent compounds and their pharmaceutical dosage forms at laboratory, pilot and large-scale production scales.
- Training and communication guidelines and programs to ensure that employees are aware of the occupational hazards presented by potent compounds and that they have sufficient knowledge of the controls used to prevent exposure.
- An effective project management system to ensure potent compounds are managed appropriately throughout the project and product life cycle, including project evaluation, R&D work, analytical, manufacturing and waste disposal.
- Technical guidelines for the design, installation, qualification, and maintenance of glove box, glove bag, and potent compound isolator systems Guidelines for the qualification of personnel using highly potent compound containment systems
„Many HPAPIs require between several and a
dozen or more process steps for synthesis.”
A History of Excellence
Since the early 1990s, these policies have been used at Evonik’s Tippecanoe facility and helped make the facility in the US state of Indiana the world’s largest contract manufacturing site for HPAPIs with the ability to run six different HPAPIs in parallel. The site has a total capacity of 170 m3 with containment capabilities down to an OEL of 0.1 μg/m3 and features multiple QC and process development lab support areas. The main T29 multi-unit, multi-product facility for HPAPIs was designed upfront to achieve effective product cross-contamination protection and worker safety.
Thanks to the efforts of industrial hygienists, maintenance teams, operations teams, quality assurance experts, engineering, and automation personnel, the site has maintained an unparalleled record for safety and containment control over more than 25 years of HPAPI manufacturing.
Evonik’s Hanau site in Germany also provides customers with process and analytical laboratories for the development and optimization of HPAPIs or ultra-HPAPIs requiring containment down to an OEL of 5 ng/ m3. The facility can produce HPAPIs on small-scale under GMP for clinical trials.
A combination of process and containment system expertise, technology portfolio flexibility and manufacturing versatility is increasingly considered essential to identify suitable CMO candidates for HPAPI and ultra-HPAPI projects. Beyond these necessities, pharmaceutical customers are increasingly making their final selection decisions on the ability of a CMO to demonstrate a history of excellence for regulatory audits, quality consistency and supply security.
















