The Future of Biopharma
How to Create a Flexible and Efficient Biopharmaceutical Production Facility
Biopharmaceutical production is in a transition phase. The recent pandemic has shown that it is becoming more and more critical to react fast and be flexible in production. The trend is towards smaller single-use bioreactors and continuous bioprocessing. In addition, digital solutions like AI-guided production planning and steering drive efficiency. In this article, we present a vision of a high-end smart biopharmaceutical production unit and a corresponding operating model.
Four factors lead to so far unsolved challenges in biopharmaceutical production. First of all, the Covid-19 pandemic has shown that sudden outbreaks of diseases require biopharmaceutical production, which is flexible, simple to scale, and readily adaptable to various technologies. New vaccines — mRNA-based or attenuated viruses — and antibodies need to be developed quickly and produced in large scale. Secondly, managing stock levels becomes more important for pharmaceutical companies in order to keep free cash flow and margins high in an environment that requires enormous investments in R&D. This becomes increasingly challenging as complexity grows along with the number of stockkeeping units (SKUs), which have increased since 2006. This is enhanced by wide variations in volume demand. Thirdly, a paradigm shift is driving the pharmaceutical industry to modular construction. Conventional facilities have proved to be costly in construction and difficult to re-purpose. Faster innovation cycles require adaptable production facilities. Lastly, policies are driving the industry towards greater flexibility. Among these policies are national immunization programs and regulatory aspects like updates to the GMP regulations and new laws, e.g., the Drug Supply Chain Security Act (DSCSA).
Increased Productivity and Flexibility
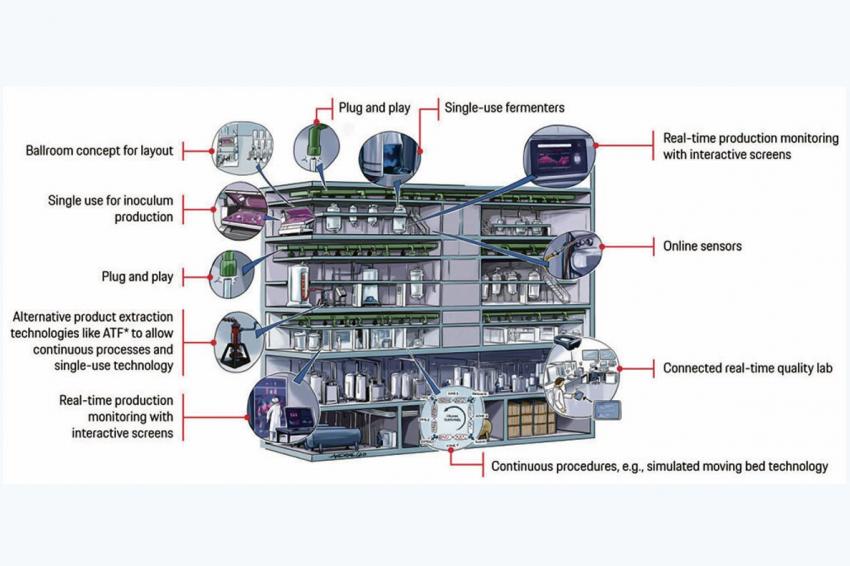
The answer to the challenges listed above lies in smart and flexible biopharmaceutical production units. The design of these manufacturing units is influenced by seven trends.
Some 45.5% of decision makers in pharma are committed to single-use bioreactors, which reduce changeover times by minimizing cleaning effort. Modeling an mAb production process in E. coli indicates that the number of batches produced per year can be increased by 117%.
While single-use technology addresses the mechanical aspects of flexibility, the plug and play concept addresses the software part of flexibility. Plug and play provides interoperability by enabling standardized communication among devices from different suppliers. Furthermore, it provides information transparency by standardization of naming of parameters and report data.
The third trend is modular construction, which is usually realized with a “ballroom” concept opposed to the classic “dance floor” concept. A ballroom is more compartmentalized. Partial areas with the corresponding heating, ventilation, and air conditioning are raised to the cleanroom class required for the respective process step. On average, the required space is reduced by 38%. Durations rather than volumes primarily govern continuous processing production. Production can simply be ramped up by extending campaigns and incorporating additional compact continuous processing units. On the other hand, it exhibits more process variability, and the process complexity increases. 25% of decision makers are committed to continuous processing in the upstream area, while 17% are committed to it in the downstream area.
Key components of the fifth trend — green design — are: reducing waste, energy, and water consumption. For example, energy can be made more green by utilizing renewable energy (e.g., solar panels) and using waste for energy. A heat, ventilation, and air conditioning system that automatically reduces air changes during non-operational hours can reduce annual utility costs by 10%.
Quality is shifting from a risk-avoiding approach to a risk-managing approach and risk-based compliance. The latter requires much more knowledge about the processes and potential risks. However, the value is a leaner and more educated approach to quality without any compromise on patient health.
The seventh trend is lean manufacturing. Margins were traditionally high in the pharmaceutical industry. As pressure continues to increase, the focus is more and more on efficiency. Lean principles have to be considered in all elements of smart biopharmaceutical production.
Overall, for the implantation of trends and technologies it is always important to consider the individual challenges and possible solutions. A decisional framework is essential for the planning of a smart biopharmaceutical production.
A Holistic Operating Model for Biopharma Production
At the start of the development of smart biopharmaceutical production, the objectives have to be specified clearly, e.g., time to launch, number of products per asset, COGS. The Porsche Consulting operating model for smart biopharmaceutical production then guides the way towards facility implementation. It all starts with the strategy and organization, which needs to be defined for the new facility. This first action field defines the vision and the mission of the new facility. Moreover, future processes and the respective organization are defined.
In the second action field, the transformation enablers need to be installed. Structured and purpose-oriented communication, which focuses on the “why,” supports the change management and motivates the employees. Competence management is important to identify and develop the skills required in the new facility. This goes hand in hand with the design of the future way of working. A specific upskilling program is essential to prepare the employees. Important transformation enablers are partners, e.g., for planning and building the new facility.
The third action field is the target picture of the smart biopharmaceutical production facility. Design principles guide the direction. These principles are derived from the specific objectives and the latest trends, described previously. They lead to a vision image like the one shown in the figure, which serves as orientation and for communication purposes.
Finally, the details need to be defined. This includes the infrastructure, buildings, and means of production. Creating a 3-D model of the facility supports the optimization of the layout. Defining the IT system early on is relevant for integrating digital solutions. In line with design principle 7, “AI-supported decisions”, installing automated production planning and scheduling increases the overall efficiency of the facility. This becomes more important if the focus is on flexibility and an increased number of products per asset, as this increases the planning complexity. Further relevant digital solutions are digital batch records and AI-supported data analytics.
In conclusion, the challenges of the modern world require new smart production facilities. When designing these, the latest trends have to be considered and a structured approach for planning has to be taken.
References to this article can be requested from the author.
Author: Till Giese, Senior Manager, Porsche Consulting, Stuttgart, Germany
Downloads
Kontakt
Porsche Consulting
Porschestr. 1
74321 Bietigheim-Bissingen
Deutschland
+ 49 711 911 12238
+49 711 911 12204