CO2 : From Foe To Friend
05.07.2013 -
Opinion - Technology that would convert carbon dioxide from a scourge on the climate to beneficial products is inching towards commercial reality and is already cheaper than carbon capture and storage.
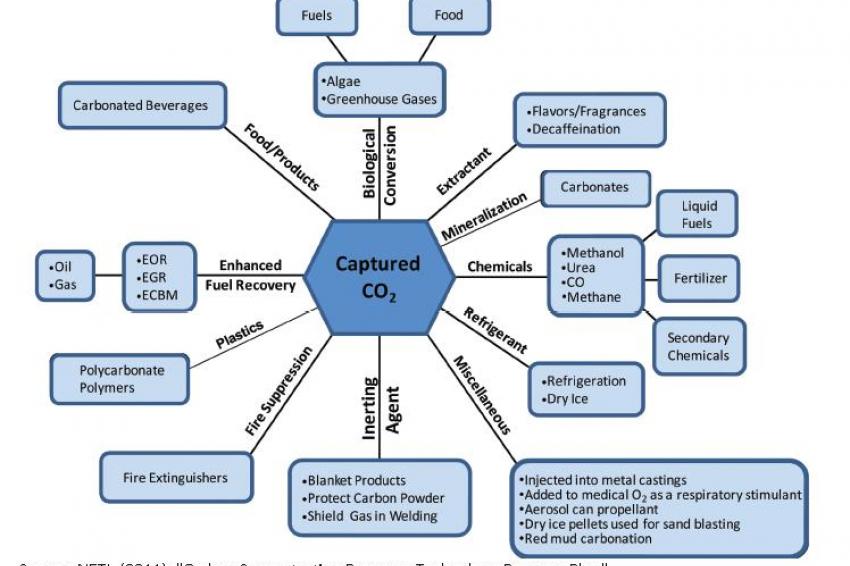
CO2 can, in theory, be converted in the making of such products as concrete, plastics and minerals. (fig. 1) There are major problems, however. Annual CO2 emissions far exceed the global market for such products, and some of these products release CO2 back into the atmosphere.
But some products such as concrete are long-lived, and costs are falling.
The technology deserves a bigger share of R&D funding compared with carbon capture and storage (CCS), the other big potential solution being considered which after a decade has still not achieved a single full-scale demonstration.
Under CCS, CO2 is stripped from flue gases and buried in underground reservoirs such as spent oil and gas fields or saline aquifers.
Enhanced oil recovery (EOR) has aspects in common with both. The CO2 is stored underground as in CCS, but like carbon conversion the gas goes to profitable use by increasing production from ageing oil wells. All are untested at full scale because of the high costs of capturing CO2 in the first place. Carbon conversion and CCS share the same initial step of separating CO2 from a diluted stream of exhaust gases.
Funds
CCS is being tested as a solution for emissions from fossil fuel-fired power plants, while carbon utilization technology, at least at the demonstration stage, has focused on industrial processes.
CCS has a longer history and has received much more funding, reflecting concerns about power plant emissions. Underground reservoirs could, in theory, store more than a century's worth of global carbon emissions.
In the U.S., the 2009 American Recovery and Reinvestment Act raised $3.4 billion for all forms of carbon capture, according to a Congressional Research Service report published last month (Carbon Capture and Sequestration: Research, Development, and Demonstration at the U.S. Department of Energy).
The funding led to 10 programs, only one of which (Industrial Carbon Capture and Storage) specifically supported CO2 utilization, with around $160 million.
Carbon utilization secured just 5% of the funding in 2010, with most of the rest going into geological storage, according to a report by the U.S. National Energy Technology Laboratory (NETL). (Carbon Sequestration Program: Technology Program Plan, 2011) (fig. 2)
Cost
Even so, carbon conversion is already far ahead on competitiveness.
McKinsey has estimated the full cost of stripping CO2 from power plant flue gases and sequestering it underground at about €40 per ton of CO2 in 2030. (Impact of the financial crisis on carbon economics", 2010)
The recovery act set a target of 2015 to "develop technologies for fixing CO2 in stable products with indirect sequestration at costs of no more than $10 per metric ton of CO2 used."
Comparing these costs per ton may be a little unfair. The flue gas of a cement plant contains a more concentrated stream of CO2 at about 15 to 30% than do power plants at 5 to 10%, making separation cheaper.
Nevertheless, the cost difference illustrates the advantage of finding an economic use for CO2.
Manufacturers of precast concrete products can "cure" a wet cement mixture with CO2 instead of steam. The cement combines chemically with CO2 to produce a stronger concrete than it would with water.
Such CO2 abatement could be sited next to cement manufacture, the second-biggest stationary source of CO2 emissions after power generation.
NETL says the process has so far achieved 90% recovery from injected CO2 and that it "should result in a net process cost of less than $10 per ton of CO2 stored". (Beneficial Use of CO2 in Precast Concrete Products, May 2013)
CO2 could also potentially be used to make organic carbonates, which go into manufacturing plastics. The main task in this case is to find a catalyst that allows CO2 to react with organic compounds at ambient temperatures.
NETL reports that a U.S. Department of Energy project has made progress in identifying suitable catalysts. (Integrated Electrochemical Processes for CO2 Capture and Conversion into Commodity Chemicals, May 2013)
Skyonic
A third potential use is conversion into carbonate minerals, which have various uses including as construction materials.
Texas-based Skyonic has patented a process to combine waste CO2 with salt and water to make products including baking soda (sodium bicarbonate) and hydrochloric acid, which can be used as a shale gas fracking fluid.
The company is one of six to progress under the Industrial Carbon Capture and Storage Projects program of the Recovery Act.
Last week, Skyonic announced that it had raised the debt and equity financing it needed to build a plant alongside a cement plant, which it expects will make baking soda and other products profitably within three years.
It is unclear how permanent the CO2 removal may be. For example, the company lists glass manufacture as one ultimate market, but glass-making vents CO2 from heating sodium carbonate.
Also the company is frank about the scale limitations of conversion of CO2. The global market for sodium carbonate and bicarbonate is equal to around 0.2% of global annual CO2 emissions, it calculates. The market is more impressive for cement and concrete, which Skyonic says could account for up to around 11% of global CO2 emissions.
At the least, carbon conversion can take a bite out of annual emissions and is important in the near term given the slow progress in commercializing CCS.