Structured Polymers Adsorb Better
Influence of Anchoring Groups on Pigment Stabilization Properties
Stabilization Differences - Acrylic AB block copolymers with different pigment adsorbing groups exhibit, depending on the particular pigments and coating systems, differences in pigment stabilization. The polymeric architecture is of equal importance for their effectiveness: High structural regularity of the block copolymers is needed to form durable and stable adsorption on the pigment surface.
Controlled Polymerization
Wetting and dispersing additives have to ensure a long-lasting adsorption on the pigment surface in order to avoid re-agglomeration of the pigments. In contrast to a statistical copolymer, where the binder compatible and the pigment adsorbing units are randomly distributed along the polymer chain, a structured copolymer provides optimized particle wetting behaviour and low millbase viscosities, due to the regular arrangement of resin compatible and particle stabilizing groups in different segments.
Among the different controlled polymerization processes available, the group transfer polymerization (GTP) technology gives access to the most defined copolymers in terms of structural regularity and molecular weight distribution, but it has some drawbacks, especially in its high sensitivity to any protic impurities in the solvent or the monomers. On the other hand, polymers obtained by reversible addition fragmentation chain transfer (RAFT) are not as defined as the ones produced by GTP, but these limitations are outweighed by the high universalism and robustness of the polymerization process.
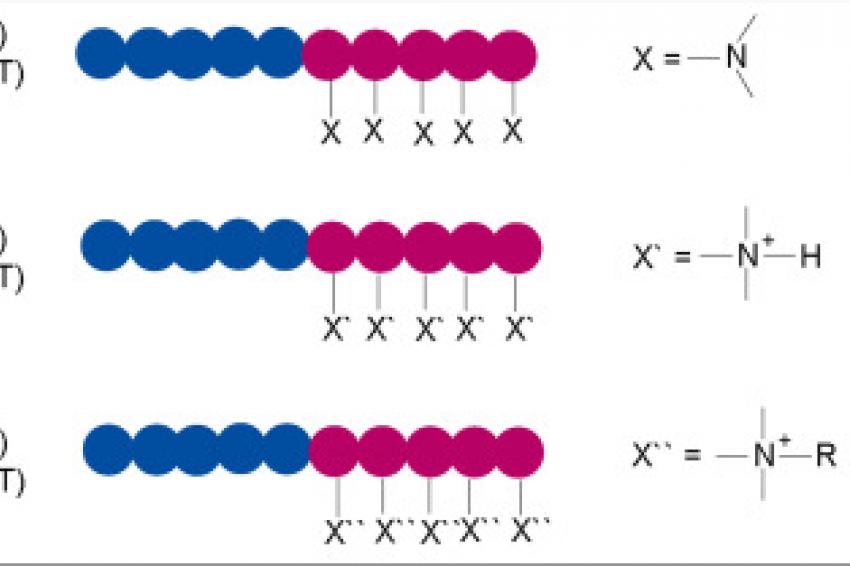
For the block copolymers, various methacrylic acid esters were selected to serve for broad compatibility, whereas a tertiary amine containing methacrylic acid ester monomer has been used as pigment interacting unit. The molecular weight distribution (MWD) of polymer 1, made with GTP, was at 1.28 remarkably lower than that for the RAFT polymer 4 (MWD = 1.69), revealing the differences in the micro structure of the polymers.
In the second step, these two polymers have been modified: the amine functionalities of polymers 1 and 4 have been either neutralized (polymer 2 and 5) or quaternized (polymer 3 and 6) (fig. 1).
Neutralized Structures Stabilize Inorganic Pigments
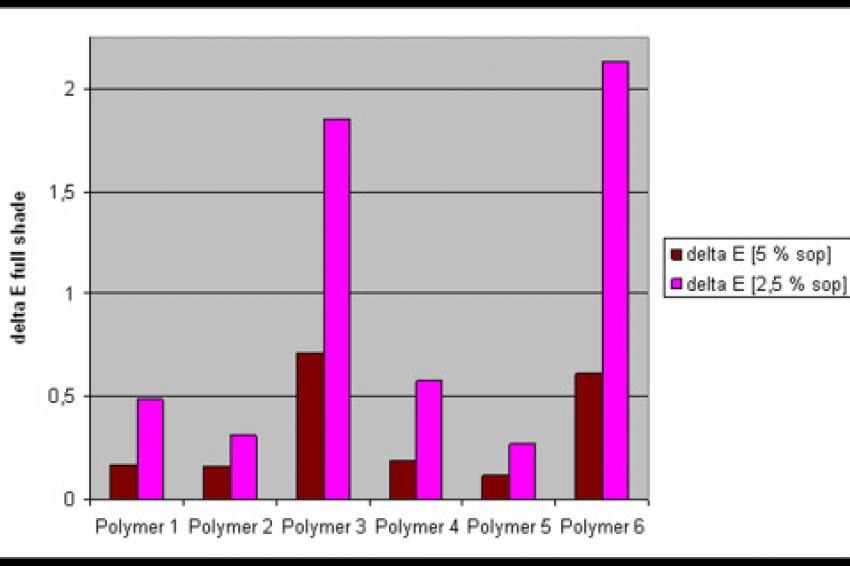
Iron oxide red pigment pastes with an acrylate grinding resin have been mixed into an acrylate/melamine let-down system. Polymers 2 and 5 showed dE (Delta E, a value that measures the distances between two colors) values below 0.3, even at a dosage of 2.5 % solid on pigment (s.o.p.), proving the ability of the ionic structure 1 as being the most effective stabilization aid for inorganic pigments like PR 101 (fig. 2). Good rub-out stability was obtained with the tert. amine functional polymers 1 and 4 at 5% s.o.p. Polymer 3 and in particular polymer 6 showed high dE values, especially at 2.5 % s.o.p. Obviously, the ionic structure 2 is not as effective as the other two pigment affinic groups in stabilizing the iron oxide red pigment.
An influence of the polymeric microstructure has only been found in the case of the block copolymers with the quaternized pigment affinic moieties, here the RAFT based Polymer 6 is inferior to the GTP made polymer 3 at the lower dosage.
Polymers with Tert. Amine Groups Interact Best With Phthalocyanine Pigments
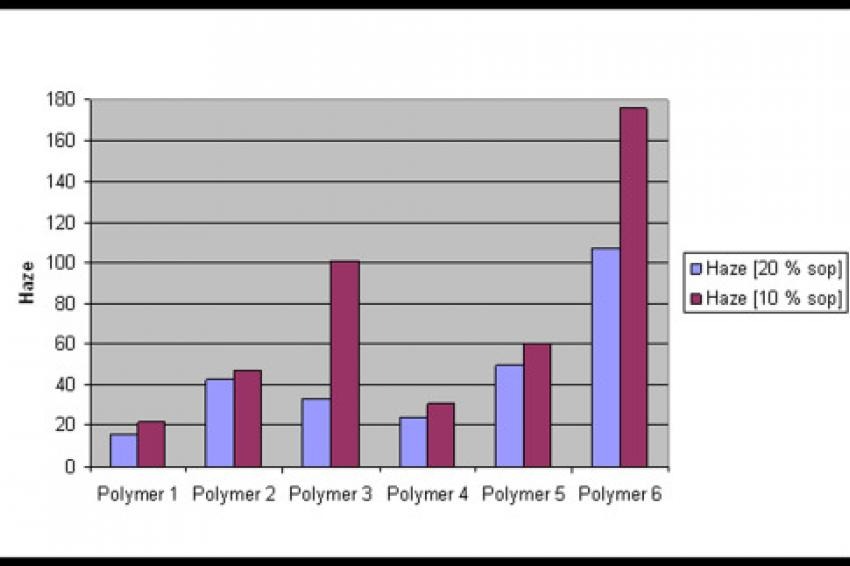
Pigment pastes based on a polyester grinding resin have been mixed into an acrylate/NCO let down system. At 10% block copolymer s.o.p., optimum stabilization properties for PG 7 have been found with polymers 1 and 4, which have tert. amine functionalities in the pigment affinic block (fig. 3). Polymer 3 and 6, which carry the quaternized ammonium groups, lead to high haze values, indicating an unsatisfactory stabilization of PG 7.
Here, besides the pigment anchoring group also the polymeric microstructure plays an important role for the effectiveness of the block copolymers: the more regular structure of the GTP based block copolymers with the concentration of the pigment affinic groups in one block leads to better particle stabilization, indicated by lower haze values.
Quaternized Ammonium Groups Best for Pigment Stabilization in CAB Systems
Cellulose acetobutyrate (CAB) is used in automotive basecoats to accelerate the drying. The higher the molecular weight of the CAB, the faster the drying behavior of the basecoat, but the more pigment destabilization processes occur, caused by a probable counter-adsorption effect of these high molecular weight resins.
Only polymers 3 and 6 with the quaternized pigment anchoring groups achieved high gloss and low haze values, indicating effective deflocculation of PR 177 in the presence of CAB (fig. 4). With GTP-based polymer 3 especially, the haze values were constantly low at around 25 units for all three dosages. Polymer 6 with the same pigment affinic group, but produced by means of RAFT, exhibited slightly higher haze values (around 50 units). The other polymers, containing either tert. amine or neutralized ammonium groups, displayed high haze values, which were strongly increasing when the dosage was minimized.
Irrespective of the pigment used, the ionic structure 2 was found in CAB coatings to be superior to the other pigment affinic groups.
In particular GTP Polymer 3, having the quaternized pigment anchoring units regularly packed in one block, led to much stronger adsorption on the pigment surface than that of CAB or other polymers, thus preventing destabilization processes sufficiently.
Summary
It has been proven that the influence of the pigment anchoring groups in AB block copolymers is crucial for the performance of a block copolymer dispersant. Polymers with ionic structure 1 as pigment stabilizing block, available via neutralization of tertiary amine groups, were found to be most effective for inorganic pigments like e.g., PR 101. Block copolymers 1 and 4, having tert. amine functionalities, can best interact and stabilize organic Pigments like PG 7 and acidic treated Pigments at very low dosages. The block copolymers with ionic structure 2 (quaternized ammonium units) are excellent candidates for deflocculating all kind of pigments in CAB containing systems.
Furthermore, the polymeric microstructure plays a key role in the stabilization of pigments: the more the pigment affinic groups are densely bundled in one block of the macromolecule, as found for the GTP Polymers 1-3, the more effective the pigment surface is covered. Thus, the ideal dispersant is a GTP block copolymer with a well balanced ratio of the three different anchoring groups in the pigment affinic block. In fact, the RAFT based block copolymers show a convincing compromise between efficiency and cost-effectiveness.
Contact
BYK Chemie GmbH
Abelstr. 45
46483 Wesel
Germany
+49 281 670 25004