Higher Yields And Less Waste
Heterogeneous Catalyst and Process for Biodiesel Production
Innovation - Biodiesel, the methyl ester of fatty acid, has been accepted as a renewable-sourced alternative to fossil-derived diesel fuel. Today, in many parts of the world, biodiesel is already blended with diesel fuel upwards of 5%. As demand for this fuel increases, so do raw material prices. Since the raw material prices account for approximately 90% of the manufactured biodiesel cost, manufacturers are constantly looking for ways to reduce their raw material cost burden.
Biodiesel is produced by the reaction of triglyceride oils: soybean, rapeseed, corn, etc. The price of these oils has been steadily increasing, to the point where they are no longer economical to use as feedstocks. Alternatively, biodiesel manufacturers are looking toward using more crude, less expensive feedstocks and also animal fat by-products. These materials often contain free fatty acid (FFA) impurities. FFA impurities result from the hydrolysis of the triglyceride oil during processing. In the case of animal fats, there is a strong correlation between oil price and level of FFA impurity (fig. 1). These FFA impurities cause problems during the base-catalyzed transesterification reaction mentioned above. The FFAs are converted to soaps by consuming catalyst, so excess catalyst must be used. These same soaps cause problems during the separation step of biodiesel manufacturing, creating an emulsion between the biodiesel and glycerol. This emulsion must then be held in storage for extended periods of time to separate completely. The necessary added catalyst, and yield loss due to emulsion often outweighs any cost benefits associated with the less expensive raw material. Therefore, in order to take advantage of the less expensive, crude, higher FFA-containing feedstocks, a method for FFA removal is necessary. We have recently developed Amberlyst BD20 technology to remove the FFA impurity by converting it to biodiesel by esterification with methanol. This reaction serves to remove the impurity without associated yield losses.
Results And Discussion
The esterification reaction of fatty acids with methanol can be catalyzed by a variety of acids, including sulfuric, hydrochloric and tungstophosphoric acids. When using these homogeneous catalysts, many potentially issues arise; including, inability to achieve full conversion and ineffectiveness with high FFA feed streams (water, the product of the reaction, tends to reduce the effectiveness of mineral acid catalysis), unwanted side reactions and by-products (sulfur incorporation into the fuel), generation of potential waste streams and need for reactors with elaborate metallurgy to prevent corrosion. We have developed a heterogeneous catalyst, with long lifetime and high activity to help circumvent these issues. At low fatty acid concentrations, the heterogeneous catalyst performs similarly to the homogeneous sulfuric acid catalyst, but at the typical 20% FFA (or higher), sulfuric acid catalysis becomes sluggish and lower overall yields are achieved. More than 60 oils, ranging in FFA content (1-100%), have been tested with this catalyst system, and in each case, the catalyst was effective at converting the FFAs to the corresponding esters.
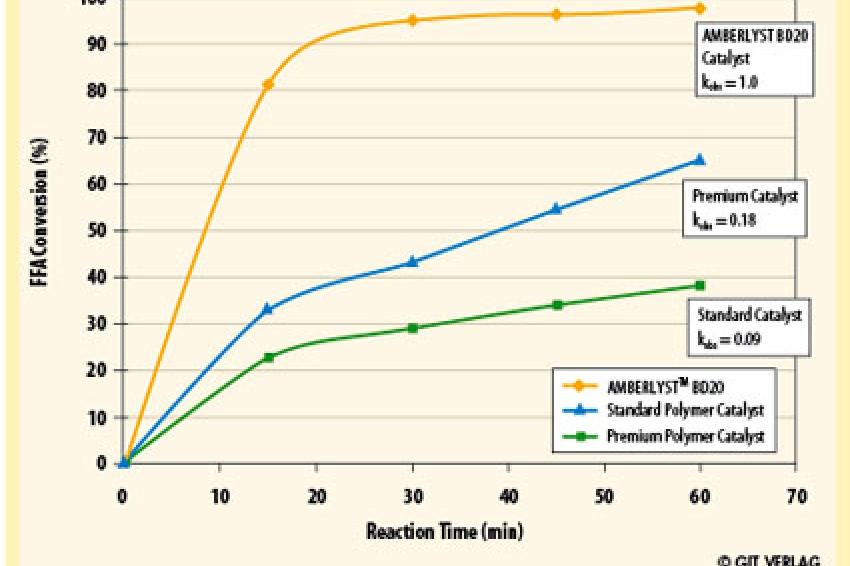
During the development of this heterogeneous catalyst, many commercial materials were tested, but in each case, the reaction kinetics were not fast enough for commercial viability (fig. 3). Since the methanol and oil are such dissimilar materials, a novel catalyst needed to be developed. The newly developed catalyst has the correct polymer structure and morphology to effectively couple these dissimilar ingredients by creating a catalytic environment amenable to both hydrophilic and hydrophobic substrates.
With the preferred catalyst defined, process research was necessary to help ensure the catalysts optimal performance. Detailed process conditions, including flow rates, reaction temperatures, reagent concentrations, etc. were optimized. The reaction can be carried out in either batch or fixed bed flow through reactor modes. The optimized conditions for fixed bed operation are 1.4 LHSV, 85 °C, and a molar ratio of methanol to fatty acid of approximately eight to one. For batch operation, 85 °C for approximately two hour reaction was adequate. Since the reaction of fatty acids with methanol to produce fatty acid methyl esters and water is an equilibrium-limited reaction, more stages, with interstage water removal may be necessary to drive the reaction to completion.
To ensure these processes were amenable to larger scale, a grant was funded by the Pennsylvania Department of Environmental Protection to build and operate a pilot unit in the scale range of 1000 gallons per batch (fig. 4). This reactor is currently located at CTI Biofuels in Pittsburgh, PA, U.S., and has been used to further optimize process conditions.
Summary And Future Direction
The Dow Chemical Company has developed a novel catalyst system to perform fatty acid esterification that:
- can perform fatty acid esterification with better efficiency than homogeneous sulfuric acid catalysis without the problems associated with liquid acids;
- has high activity for esterification (rapid, quantitative conversion at lower temperatures);
- has high selectivity (no ether or olefin formation);
- has a long lifetime (>3000 BVs), catalyzes complete conversion of FFAs into FAMEs (99+%) in all oils and fats; and
- allows the biodiesel industry to benefit from flexible, lower cost feedstocks with higher yields and less waste.
As the need for alternate fuel sources continues, the use of inedible, renewable feedstocks will become critical. The technology described above will allow for the use of crude, inedible materials as feedstocks for the conversion to biodiesel fuel.