Gartner findings: No Prospective for Global Solution
Assessing Track-and-Trace and Serialization Legislation for Life Science Companies
Track-and-trace and serialization legislation for life science companies is gaining momentum around the world. Supply-chain leaders and operations managers must consider compliance with new legislation while examining the broader business facilitating opportunities.
Health-care delivery providers and manufacturers are working together to develop end-to-end, value-based supply chains. At the same time, global track-and-trace and serialization laws are truly embracing the principles of end-to-end supply chains. Supply-chain leaders and operations managers must understand the urgency to comply with these new laws. This also presents opportunities to innovate across other supply-chain processes and form more direct relationships with patients, which can improve demand planning, brand reputation and profit margins.
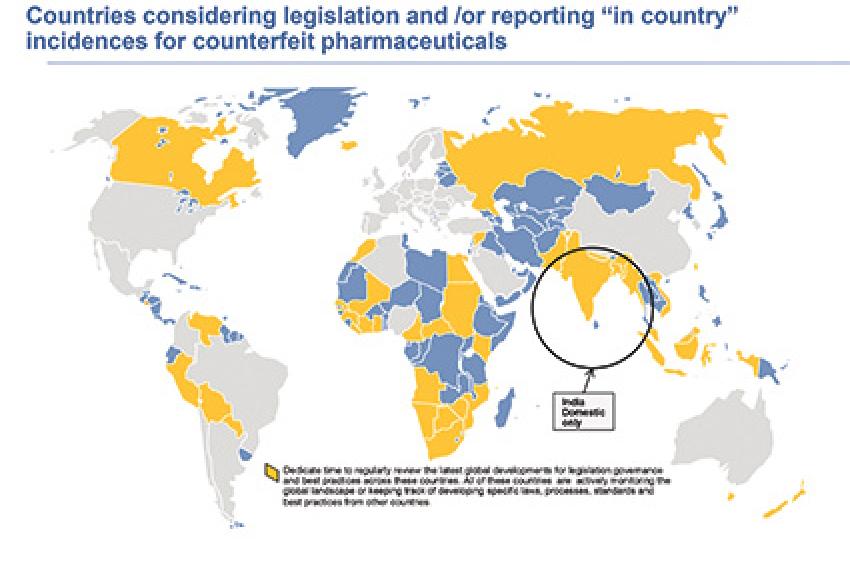
The global proliferation of counterfeit pharmaceuticals that pose a serious potential threat to patients has recently led to a significant increase in mandates for track-and-trace and serialization. High levels of theft, diversion, fraud and goods lost in transit adversely affect supply-chain performance, revenue and brand reputation.
Supply-chain stakeholders and regulatory bodies have been working together to develop mandates and technology platforms to support these track-and-trace and serialization processes. In Europe, Africa and parts of Asia, product authentication is now the primary mode of protecting patients.
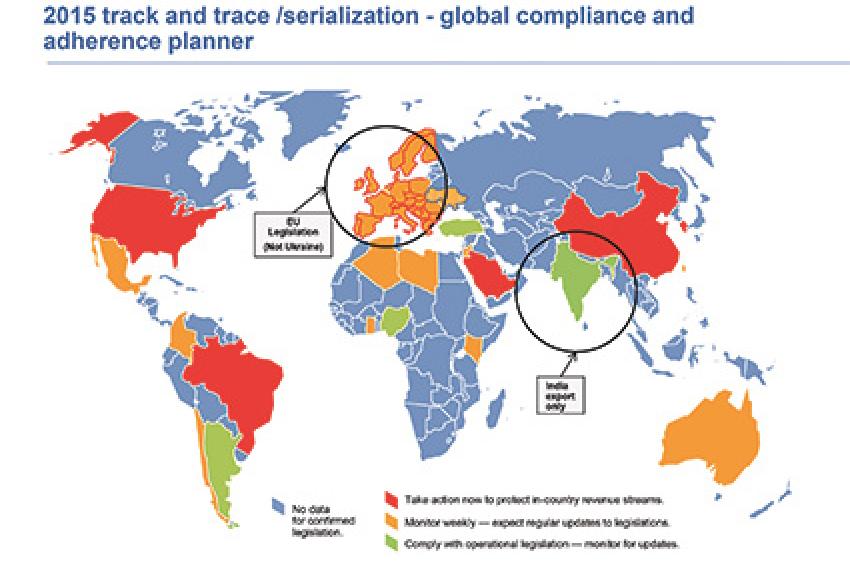
With no global governance models in place, individual country laws have evolved independently and become fragmented. These laws have staggered compliance time frames, including some with requirements for this year. To help our clients understand their obligations, Gartner has prepared research notes - highlighted below - to explain the legislation.
Worldwide
In "Assessment of Track-and-Trace and Serialization Legislation for Life Science Companies, Worldwide," Gartner provides a broad overview of emerging laws, which is further expanded upon in a series of research notes focused on individual regions. Brazil, China, Saudi Arabia, South Korea and the US all require immediate decision-making for deployment of track-and-trace and/or serialization solutions. The legislation in these countries will also affect key supply-chain stakeholders.
It's important to act quickly and look beyond compliance to explore the wider business benefits that could result from serialization and track-and-trace solutions, such as enhanced supply-chain visibility, optimized packaging changeover times, returns and recalls integration, data complexity reduction, multi-enterprise integration and connectivity across the entire health-care value chain.
Enhanced supply-chain visibility is one of many capabilities for business facilitation through the emerging reference models for product security. "Use Serialization Deployments to Enhance Your End-to-End Supply Chain Visibility" discusses these benefits and capabilities.
United States
The 2013 Drug Supply Chain Security Act marked a turning point for life science companies. They began to look more globally at how the legislation would affect both their near-term and longer-term business strategies for brand protection and track-and-trace and serialization requirements. "Assessment of Track-and-Trace and Serialization Legislation for Life Science Companies, United States" explores the implications for manufacturers, re-packagers, wholesale distributors, dispensers and third-party logistics providers.
The law provides a 10-year compliance window for a fully electronic and interoperable network. It also establishes a comprehensive set of near-term mandates as well as longer-term guidance time frames for further ratification of additional capabilities in consultation with stakeholders and industry.
Europe
European serialization mandates are expected in 2018. Europe defines a special classification for "falsified" medicines. The European Commission and industry supply-chain stakeholders have developed laws, processes and a cloud-based technology platform to protect patients from falsified medicines. The focus is on product authentication at points of dispensation, which is different from track-and-trace laws in other countries. Additional requirements and capabilities are being discussed as part of a schedule of delegated acts that will eventually become law. "Assessment of Track-and-Trace and Serialization Legislation for Life Science Companies, Europe" explores this series of laws, regulations and processes.
Eastern Europe, the Middle East and Turkey
Four countries have established processes in this region. "Assessment of Track-and-Trace and Serialization Legislation for Life Science Companies, Eastern Europe, the Middle East and Turkey" explores supply-chain operational and strategic requirements and opportunities for these countries. Turkey's Ilaç Takip Sistemi (iTS) is considered the most mature example of deployment of a multi-stakeholder track-and-trace system for pharmaceutical products. As well as ensuring patient safety, iTS can deliver additional business outcomes such as fraud prevention, inventory control, optimized returns and IT interconnectivity.
Saudi Arabia and Jordan have been developing specific guidance legislation, with recommended compliance mandates beginning in March 2015 and 2017, respectively. Ukraine has been drafting legislation and undertaking pilots with key stakeholders and centralized repositories.
South America
"Assessment of Track-and-Trace and Serialization Legislation for Life Science Companies, South America" focuses on Brazil's comprehensive phased mandates and immediate compliance requirements in 2015. Argentina has had product security mandates focusing on end-to-end supply-chain traceability and selective tamper-evident product integrity in place since 2011. Evolving operational models in Argentina and Brazil are influencing other countries in the region to develop their own laws and systems to secure drug products.
Asia/Pacific
In "Assessment of Track-and-Trace and Serialization Legislation for Life Science Companies, Asia/Pacific," we discuss varying levels of development across the region. China has comprehensive legislative requirements, with important compliance milestones set for 2015. China's legislation has differentiated requirements, some of which are unique. Operating principles are full track-and-trace, with stakeholder verification and customer authentication ability. India has regulations covering packaging levels for export markets only. South Korea has legislation in process, with phased compliance requirements that start in 2015.
Africa
In "Assessment of Track-and-Trace and Serialization Legislation for Life Science Companies, Africa," we explain that track-and-trace and serialization laws have yet to emerge in Africa, despite many years of reported seizures and incidents of counterfeit medicines. The region presents unique challenges. Manufacturers and regulators have adopted product authentication through mobile connectivity to patients as the primary way to begin tackling this significant threat to patient safety.