Vaccine Filling at the Highest Level
Recent studies show that contract manufacturers for the pharmaceutical industry, so-called CDMOs (contract development and manufacturing organizations), have so far been little involved in the production of vaccines. This is changing with the outbreak of the Covid-19 pandemic.
There are two reasons for this: On the one hand, vaccine developing companies often do not have the capabilities of commercial production and, on the other hand, the current pandemic situation requires the rapid supply of very large numbers of vaccine doses worldwide, i.e. commercial production on a very large scale. Leading CMDOs like Aenova Group can support these challenges as a "full service provider" - from small scale manufacturing of materials for clinical trials, technical transfer to scale up of high volume production of fill and finish of biologics.
Fill & Finish of Vaccines
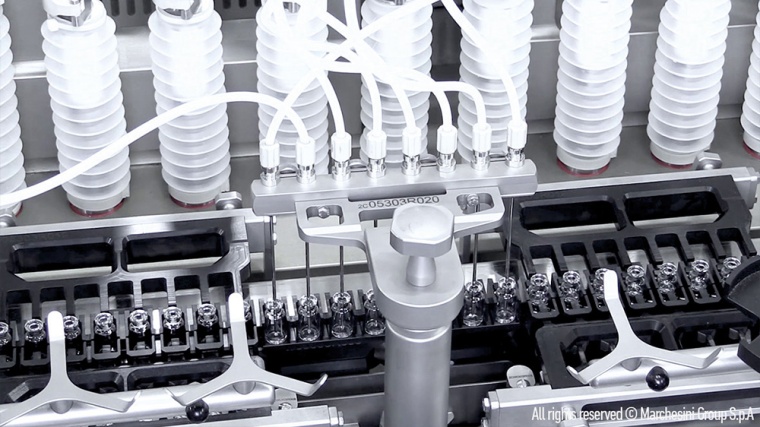
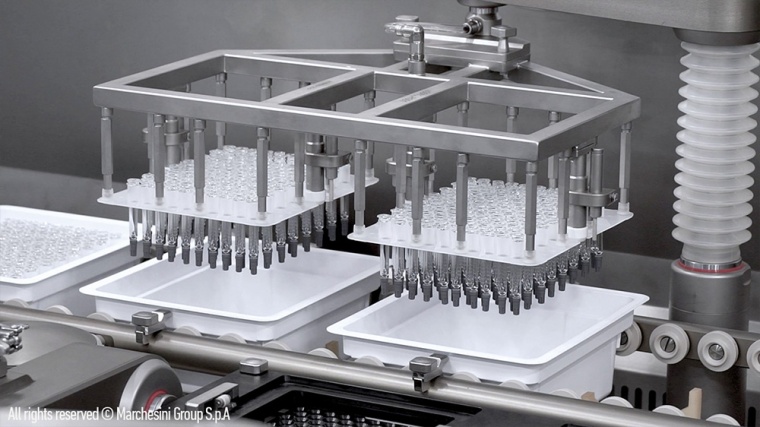
In order to expand its capacity for the production of vaccines against the SARS-CoV-2 virus, Aenova has just launched a major investment program that includes the installation of further state-of-the-art equipment for aseptic production. The focus is on a completely new "fill and finish" area for vials and prefilled syringes (PFS) with high-speed filling lines including compounding at the Aenova site in Latina, Italy. In the future, more than 80 million vials (glass and plastic) and over 180 million prefilled syringes can be produced there per year. Aenova is also planning a further capacity expansion with up to three additional high-speed filling lines.
Lyophilization and Aseptic Powder Vials: from Adenoviruses to mRNA
As a leading solution partner for sterile dosage forms, Aenova has many years of experience in the production of sterile injectables - from pre-filled syringes and ampoules to liquid vials, lyophilized vials and aseptic powder vials (powder filling in vials), also for biological preparations and Bio Safety Level 1 and 2 (BSL) vaccines: Expertise in vaccines ranges from mRNA, DNA, viral vectors, protein subunits, virus-like particles, to inactivated vaccines.
Thus, vaccines can be filled with adenoviruses such as those from AstraZeneca and Johnson & Johnson or Sputnik V, as well as with messenger RNA such as those from Pfizer/BioNTech and Moderna.
The new sterile area is based on state-of-the-art technology: the aseptic filling process is fully automated, and the multi-purpose machine can process vials as well as PFS products in the preferred formats of 0.5 to 10 ml for prefilled syringes and 2 ml to 10 ml for ready-to-use vials.
Quality control, packaging and labeling including serialization complete the scope of supply.
Many Years of Experience in Contract Manufacturing
CDMOs such as the Aenova Group can offer very clear advantages: in addition to many years of experience in a wide range of contract manufacturing disciplines, these are above all the end-to-end services from development, clinical studies I to III, through to large-scale commercial production. The globally active company with 14 manufacturing sites for all common dosage forms can point to many national and international certifications and approvals, such as that of the US FDA for the three sites with sterile production.
Seven Competence Centers for Development, Analytics, Clinical Trial
Supply management, regulatory support and further technology centers for seamless tech-transfer and product-life-cycle management complete the service range around the commercial production of large volumes.
As an added bonus for Aenova, the company can process live attenuated vaccines on state-of-the-art BSL-2 classified freeze-drying equipment.
Contract manufacturers like Aenova can also score points with their experience in supply chain and regulatory controls, in addition to both technical and process expertise for GMP-certified facilities and specialty manufacturing areas - diverse subject areas for which specialist know-how and years of experience are prerequisites. That's something many of Covid-19 vaccine developers can't boast.
At the same time, a key strength of CDMOs is that they always keep a very close eye on manufacturing cost efficiency, production quality and delivery reliability. This is the only way they can position themselves as reliable partners for their clients.
From Development to Large-scale Production
Sterile injectables play a key role in the development of new drugs, as demonstrated by the SARS-CoV-2 vaccines. Aenova offers services for all common parenteral formulations for intravenous, subcutaneous, and intra-muscular use, as well as the development of aseptic fluids up to OEB 5. The demanding cold chain required for filling sensitive products such as mRNA- or DNA-based formulations is mapped, and all microbiological tests can be performed in-house.
Thus, the Aenova Group offers its customers the complete service of a one-stop store - from development to scale-up of commercial production.
Spnsored by