Disinfectant residues in cleanrooms
-
Within the current draft annex, version 12, the references to cleaning and disinfection have been expanded. The terminology of “cleaning” has been replaced with “cleaning and disinfection”. The draft text notes that “for disinfection to be effective, prior cleaning to remove surface contamination should be performed”. As well as efficacy concerns that a residue may inhibit the efficacy of the disinfectant, or support microbial growth, residues can also cause a safety problem, slippery floors or transfer to product contact surfaces. There could be functional issues created, as well as aesthetic issues of the facility not looking “clean”.
Disinfectant residues
Cleanroom residues can be generated by the process, raw materials, certainly by people but a significant contributor to the residues in a cleanroom are the disinfectants themselves. Many common and well used disinfectants, leave significant residues on a surface, which can subsequently have a detrimental effect on the effectiveness of the disinfectant used. This is acknowledged in draft Annex 1, “Cleaning programs should effectively remove disinfectant residues.”
There are currently no approved or validated methods for assessing the amount of residue on a non-product contact surfaces. Many or even most facilities will conduct a visual test for residues on non-product contact surfaces only. As previously mentioned many of the commonly used disinfectants in cleanrooms, can themselves leave significant residues on a surface. This can manifest as “sticky” surfaces, smeared windows, yellowed equipment; potentially leading to corrosion and damage.
There is no definition or standard of what constitutes a “no” or “low residue” disinfectant. However, there are claims made on disinfectant advertising that a disinfectant is either “no” residue or “low” residue. The data to support this is usually a simple residue on evaporation test.
The European Pharmacopoeia has a residue on evaporation test which can easily and cheaply be used to quantify the amount of non-volatile residue left by a solution. The test method simply requires 100 ml of the solution to be boiled to dryness in an evaporating basin of known weight.
EP Residue on Evaporation Method
- Evaporate 100 ml of test substance to dryness in a water bath and dry at 100 – 105 °C for 1 hour
- Weigh container after drying and subtract weight of the original container
Two chemicals used as disinfectants which do have an EP monograph are Isopropyl Alcohol and Ethanol, these have limits for residue on evaporation. The limit for 99 % Isopropyl Alcohol is 20 ppm and 25 ppm for 96 % ethanol. Both of these products would be universally accepted as leaving no residue on a cleanroom surface. So, a product which leaves a non-volatile residue of less than 25 ppm could be classed as no residue?
The only other commonly used cleanroom disinfectant which leaves a residue as low as alcohol is hydrogen peroxide. Hydrogen Peroxide (H2O2) breaks down to water and oxygen on a surface, the reaction taking place is 2H2O2 → 2H2O+O2. Test worked carried out on Contec’s Hydrogen Peroxide confirms this with ROE results between 4 ppm and 7 ppm. However, this cannot be assumed for all hydrogen peroxide solutions. It is a solution in equilibrium so different grades of hydrogen peroxide contain different amounts of stabilisers which can contribute to the amount of residue left behind. Blended disinfectants which contain alcohol or hydrogen peroxide with other chemicals can also leave considerable residues. Table shows the residue on evaporation levels for a range of cleanroom disinfectants manufactured in both the USA and Europe.
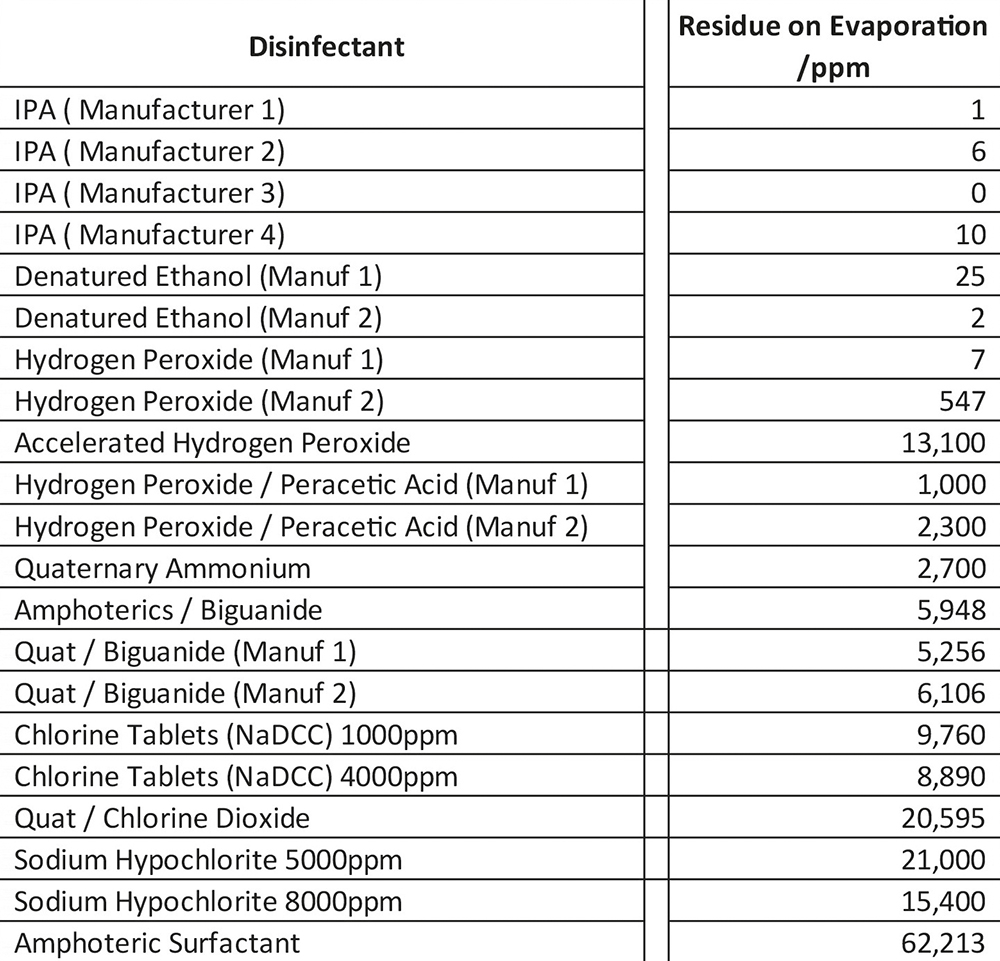
As can be seen from the results the level of residue for the different active ingredients can vary significantly, from products leaving approximately 400 ppm up to products leaving 62,000 ppm. The table also shows, there can be a difference for disinfectants containing the same active ingredient, this will be down to the concentration of the active ingredient in the product, the amount of stabilisers required and potentially the addition of preservatives, pH adjusters or odour mitigators.
Cleanroom disinfectant residue on a surface
Disinfectant residues can take many forms, clear, white, yellow, pink, solid, gelatinous, crystalline, powdery, or sticky. The visual appearance of the disinfectants on a surface may not always match the amount of residue shown on a residue on evaporation test. The method of application of the disinfectant will also affect the residue.
Table 2 shows the build up of residue of different cleanroom disinfectants on a surface. Highly polished mirrors were used as previous work carried out showed that reflective surfaces show residues more easily.
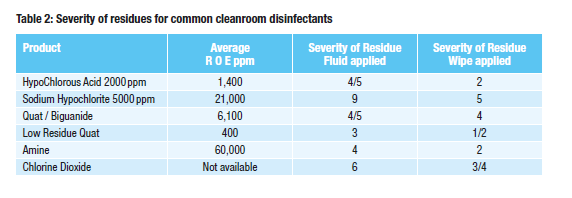
Each mirror had 2 mL of a common biocide/sporicide added each day to the centre of a silicone ring (50 mm internal diameter) for a total of 14 days. The liquid was left to dry, no attempt at wiping the liquid was made. Liquid was added via a measuring syringe. Results were on a scale of 0 – 10 with 0 being no residue or marks left and 10 being large, thick, highly visible residue left. This process was chosen as we wanted to create worst case residues where a disinfectant had been left to pool for example. The residues left for the different disinfectants can been seen in photograph A of figure 2.
The disinfectant was also applied for 12 days using a wiping method one Polyester/cellulose dry wipe was used per mirror. The wipe was folded into quarters and then the product was sprayed onto one side five times (roughly 5 mL of each product added per wipe) at a distance of 10 cm each time. The product was applied, via a single wipe, down the centre of each mirror, twice daily for 12 days — a total of 24 wipes per mirror. This more closely reflects how a product would be applied routinely in the cleanroom. The residues left for the different disinfectants can be seen in photograph B of Fig. 2.
Residues on different surfaces
The appearance of the residue on different surfaces within the cleanroom can also look lesser or greater for the same amount of residue dependant on the characteristics of the surface itself. Fig.1 shows the same amount of disinfectant applied to five different common cleanroom surfaces.
All the sample surfaces were cleaned by spraying with Contec Denatured Ethanol and wiped down with a dry polyester wipe. The samples were tested in triplicate. Every working day for four weeks the sample surface was sprayed 3 times from a distance of approx. 30 cm with Contec ProChlor. The disinfectant was left to dry and allowed to build up on the surface. The residue left from Contec ProChlor, a hypochlorous acid product, is a calcium salt, which appears as a white powdery residue. The residue on evaporation for Contec ProChlor is between 1000 – 1500 ppm. The photos in Fig. 1 show this amount of residue can look very different on the different surfaces, dependant on its colour, smoothness and reflectiveness.
Highly reflective surfaces such as glass and polished metals will show the residue more significantly, although the amount of residue there will be the same. This means a purely visual check of a residue may not be sufficient. A glance at the windows in a cleanroom will very often quickly reveal if there is a problem with residues on other surfaces as well, as most residues can be clearly seen on glass.
Not all residues are created the same
As table 1 showed many common disinfectants leave a residue to greater or lesser degrees. Draft annex 1 states that the cleaning process should be validated so that it can be demonstrated that it can remove any residue that could create a barrier between the sterilising agent and the equipment surface. The validation should also prove that residues are removed that could contaminate the product with either chemical or particulate contamination.
Consideration should be given not just to the amount of residue left but how easily the disinfectant residue is to remove from the cleanroom surfaces. Old and scratched surfaces may also influence residue removal. Some residues are free-rinsing and easy to remove, others are “sticky” and waxy and can be difficult to remove from a surface. This can also change over time, a residue easily removed immediately after the contact time might not be so easily removed weeks later. If an immediate rinse stage is not to be used, work would need to be carried out to show how long a residue can be left before removal becomes more difficult.
In order to define this for a cleanroom disinfectant used in Contec’s own cleanrooms a cleaning phase was carried out at each stage of the residue build up work. When the disinfectant was sprayed onto the surface for four weeks, the salt residue was visible from week 2. Previous studies had shown that the residue could easily be removed immediately after use with both water and IPA. We wanted to validate that we could use the product without a residue removal stage for a month, so work was carried out at intervals to see if the residue remained free-rinsing.
After three weeks of residue build up the visible salt could be removed with an IPA wipe from all surfaces. However, when this test was carried out after four weeks of residue build up the salt could be removed from all surfaces but not the 304 grade stainless steel. At four weeks the salt became insoluble and significantly more difficult to remove. This meant the recommended use for this disinfectant, was either remove immediately after contact time by wiping-to-dry or removed with water or IPA or with a residue removal stage at three weekly intervals.
Other factors
This starts to give an insight into how much validation work maybe required to meet the requirements of the new draft annex 1 if a “no-residue” disinfectant is being used. A lab based, residue on evaporation test, will give a quantitative result of how much residue a disinfectant will leave and if it is above 25 ppm per 100 ml further validation work would be required.
The method of application of the disinfectant will need to be taken into account, as this affects the amount of residue left on a surface in use. This could be seen by our qualitative study, further work needs to be carried out, to find an easy-to-use, robust method of quantifying a residue on a surface in normal use. This could possibly be done by conductivity measurements, UV contrast chemicals and computer imaging, the addition of luminescent bacteria and luminometer.
The work needs to be carried out on the different surfaces in the cleanroom as the appearance of the residue on the different surfaces varies dependant on the smoothness and reflectiveness of the surface.
If an immediate wipe to dry or rinse stage is not used at the end of contact time, the amount of time the residue can be left before removal needs validating as a residue that can be removed after a week may not be able to be removed after a month. The roughness of a surface will also influence how easily a residue can be removed. The choice of cleaning solution will need to be validated, whether WFI, alcohol or a detergent (surfactant) solution works best. This might not be the same for all disinfectants that you are using.
If we can quantify the amount of residue on a surface, then a pass criteria for residual removal can be stipulated not just that a surface looks visually clean. Consideration would need to be given to the pass criteria. Is the residue removal only successful if the levels remaining on the surface are below 25ppm or can a higher limit be justified? At what residue level does there start to be some detrimental effect on the disinfectants used, or risk of carry over onto product contact surfaces.
There is certainly more work to do on understanding, quantifying and removal disinfectant residues — watch this space for further updates.
Author










