Here To Stay
The Significance of Catalysis in the Pharmaceutical Industry
A Challenging Business - The medicines supplied to patients are the final result of a very lengthy process (commonly about 10 years) and a huge investment both in monetary terms (exceeding $1 billion) and in staff effort (a drug project of decent size will easily involve hundreds of experts covering various scientific, technological and clinical areas). In spite of the resources available to at least the major pharmaceutical companies including all the experience and knowledge, the business continues to be haunted by a very low success rate. Thus, out of 10 projects taken into pre-clinical development, on the average merely one will survive all the way to the market.
The Drug: A Complicated Piece of Construction
Looking into the manufacturing process for making a drug ready for use by patients, two discrete stages will become visible. Firstly, the creation of what is commonly known as the active pharmaceutical ingredient (API), which is the chemical compound - often an organic molecule of medium to high structural complexity - where the pharmacological effect resides (this assumes that we are focusing small molecules as opposed to biomolecules, such as antibodies or proteins).
At first glance, you might think this is all it takes to produce a drug product, but the fact is that the API is just an intermediate on the way to the final drug. Instead, a second step is required where the formulated product is achieved, that is when the active compound is "dressed up" as a tablet, an injectable, an ointment or in inhaled form. In short, this involves adding various components described as excipients which will provide features such as stability, powder characteristics, taste masking etc. In other words, drugs delivered to patients represent highly sophisticated materials that have been achieved in close cooperation between chemistry, engineering and pharmacy to arrive at a final product that has to prove its efficacy and safety in rigorous clinical and toxicological studies.
Sustainability - A New Paradigm in the Chemical Industry
One of the elements in the renowned Principles of Green Chemistry is the area of catalysis. Alongside the other 11 topics listed, catalytic chemistry is seen as a major contributor to ensure that the technologies applied and the way of operating is following the philosophy of reducing the footprint of mankind in the environment. In this sense, catalysis will also play a key role in creating a sustainable society going forward. The aim will be to use the available feedstock of raw materials as efficiently as possible, hand in hand with an outspoken ambition to minimize waste emanating from chemical processes, which obviously can be, at least in part, achieved by recycling of spent solvent and recovery of catalysts.
Various measures to describe the efficiency of chemical reactions, for example the frequently utilized E-factor (defined as quantity [kg] of feed materials per quantity [kg] of product out), is an extremely good eye-opener that will help draw the attention to bottlenecks and weaknesses in a process, regardless if it is constituted by a single step or a multi-stage procedure, which then can be addressed and hopefully improved.
Homing-in on Synthetic Chemistry: Catalysis as a Major Tool
While catalytic transformations and processes have been a mainstay in the chemical industry for a very long period of time, it is only fair to say that pharmaceutical companies generally speaking have acted more as laggards. The root causes for this are manifold, but a major factor has undoubtedly been the lack of drivers forcing the business to change gears and start applying catalysis in a more consistent manner. Key contributing factors in explaining this reluctance are arguably the special circumstances under which pharma operates, namely on a heavily regulated, monopoly-type market where huge corporations dominate the playground by virtue of their strong IP and patent protection for own products. This situation effectively eradicates all competition, in the ideal case at least until the drug goes generic, and, hence, the need for implementing more challenging and complex technologies (of which catalytic chemistry is one) by the sheer fact that there is no or only limited competition to take into account.
Nowadays, such attitudes have changed thoroughly under the influence of multitudes of reasons, for example: cost of goods where authorities, markets, and payers are demanding lowest possible prices for ethical drugs; a general buy-in across the business that manufacturing processes should utilize best methods and techniques available at a given point in time; a realization that many of the complicated molecular architectures represented by today's drug compounds cannot be achieved sustainably by "traditional" approaches; and, last but not least, due to the aforementioned commitment to green and sustainable processes.
We are now witnessing how the pharmaceutical industry at large is keen on a broad application of both conventional catalytic technologies as well as front-line novelties to solve their problems. Many of the current flagship products in commercial use rely heavily on catalytic transformations. Against this backdrop, and by virtue of the rich repertoire displayed by the full breadth of catalytic methodologies at hand, the standing of catalysis in pharma R&D and large scale manufacture has never been as strong as today. A few examples will serve as nice illustrations in demonstrating the versatility and the power of catalytic technologies.
Catalytic Breakthroughs - Illustrative Case Stories
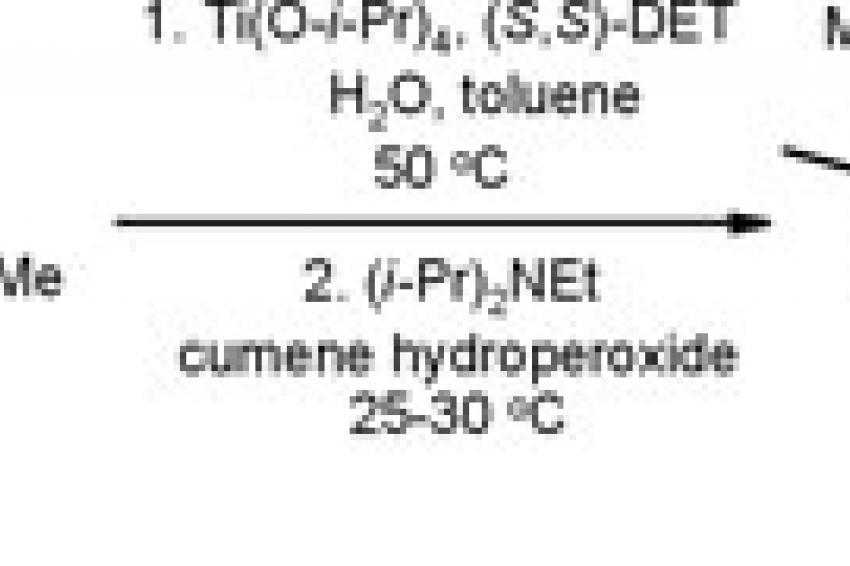
One of the few catalytic transformations operating in an asymmetric mode that has actually survived through all phases of R&D and made it onto the market is the manufacture of esomeprazole, the active ingredient in AstraZeneca's antiulcer drug Nexium. Best currently available estimates give the number of commercial processes relying on asymmetric catalysis to less than 20, which, however, does not reflect a poor utilization of this approach but more so that most of the industry's drug projects will fail along the path before reaching launch as was already alluded to earlier.
The unique features with this method (fig. 1) is the unsurpassed performance in terms of chemical yield and stereochemical purity achieved by using a groundbreaking combination of a titanium catalyst and a simple amine reagent (Hünig's base). Reliability and robustness are other key characteristics of the process that has been thoroughly validated by producing tens of tons of material per annum for more than a decade.
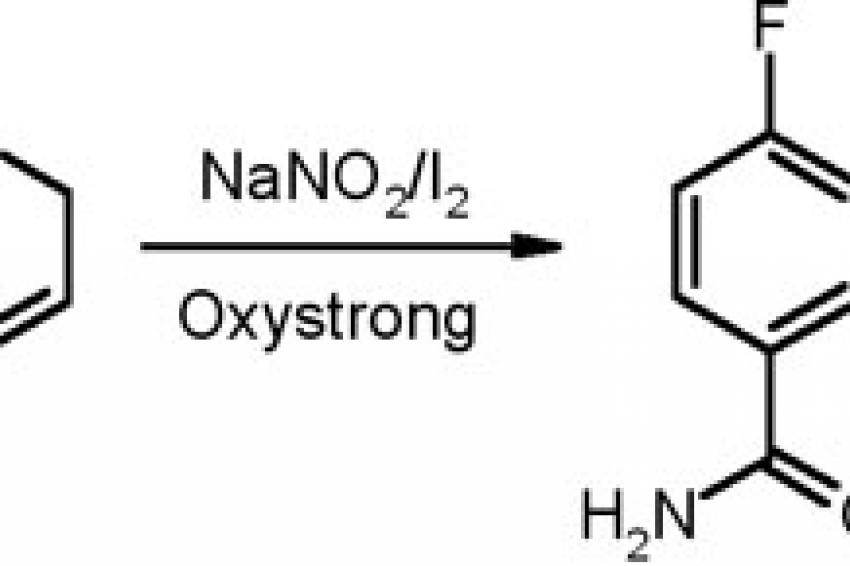
The fact that catalysis can be obtained not only with various types of metals is nicely illustrated by the olefinic nitration, constituting a critical step in the sequence leading to robalzotan, a compound developed for the treatment of depression which, unfortunately, had to be discontinued. In this case, elemental iodine (I2) is applied in order to activate the double bond before expulsion as HI which is accountable for re-generating the double bond. While the literature procedure required a huge excess of I2, this created major problems in the work-up, and therefore a catalytic alternative had to be constructed.
The clue to this problem was realizing that in order to install a catalytic cycle, the iodide formed as part of the process had to be re-oxidized to I2, and this was found to be conveniently achieved by adding an oxidant in situ (a mixture of per-acetic acid and hydrogen peroxide in water/acetic acid, marketed under the brand name Oxystrong 15). With this profound process modification the loading of I2 could be reduced from super-stoichiometric amounts (3 mol eq.) to just 20 mol-%, which, concomitantly, enabled a much less burdensome isolation of the nitrated intermediate (fig. 2) in the robalzotan synthesis in considerably enhanced yield (70-75% vs. 45%).
Over the past 30-40 years, we have been witnessing a formal explosion of coupling methodologies - for example Heck, Suzuki, and Buchwald-Hartwig - which all in all have provided valuable tools that allow ever more complicated structures to be synthesized in a straightforward and direct way. Originating from academia, many of these new procedures have withstood the test on large scale, albeit some only after considerable modifications and optimizations.
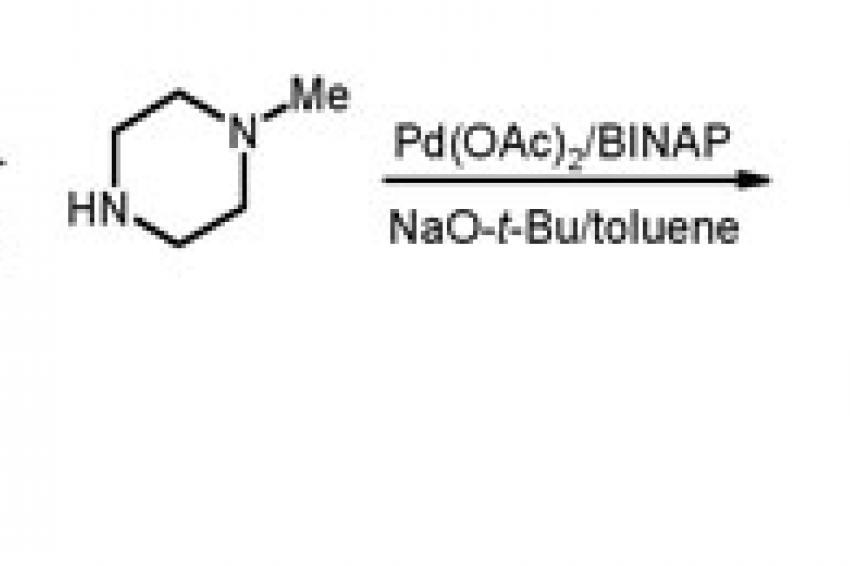
A case in point is the AR-A2 project (abandoned in phase II due to side-effects), which was aimed at developing a novel antidepressant (profiled as a 5HT1B antagonist), where one of the critical steps was the attachment of a heterocyclic moiety onto the core part of the molecule, a chiral aminotetralin. Thus, is was shown that the catalyst loading of this Pd-catalyzed transformation could be reduced to <1 mol-% and by further refinements of the reaction conditions, the overall yield was close to quantitative as demonstrated on 125 kg pilot plant scale (fig. 3).
Outlook: The Direction of Catalysis in the Future
Against the backdrop of today's already established position as a key technology capable of ensuring that challenging chemical structures are successfully delivered in an efficient manner, the catalysis field is, most likely, bound to undergo further significant and pronounced developments going forward, as seen from a pharmaceutical industry perspective.
A confirmation of its continuously strengthened role as a tool of increasing versatility is the fact that estimates indicate that one step out of an average of eight required to synthesize an API will be catalytic. While existing methodologies such as coupling reactions, transformations in heterogeneous systems and asymmetric hydrogenations among others will stay on as the dominating ones, new additions will be seen in changing stoichiometric reactions to their catalytic counterparts. Thus, it can be hoped that such standard reactions as de-methylations, amide formations and preparation of certain fluorine-containing motifs will see new opportunities in the near future.
An interesting development can also be expected at the interface between chemo- and bio-catalysis (based on enzymes), possibly with opportunities for organocatalysis (small organic molecules as catalysts) which constitutes an area showing a strong upwards trend. Moreover, switching to less-precious metals (for example to Fe and Cu) is something that might boost catalysis at large, if nothing else then from a cost point of view.
Finally, the emerging topic of predictive catalysis that will allow the user to quickly home-in on the best catalytic system has a lot speaking for itself as it has the attractive feature to enable process development to occur much faster. The same argument can be applied to catalysis in flow systems - an area of considerable hype nowadays - as this brings with it a fast progress from laboratory experimentation to a large scale manufacturing scenario. At any rate, catalytic technologies are here to stay and their importance will only increase in the foreseeable future.
References are available from the author upon request.
Contact
AstraZeneca
Byggrad 2/4
15185 Södertälje
Sweden
+46 8 55327063
+46 8 55324276